Gut microbiota and tuberculosis
- PMID: 40860431
- PMCID: PMC12371273
- DOI: 10.1002/imt2.70054
Gut microbiota and tuberculosis
Abstract
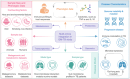
Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB), remains a significant global health challenge. Recent advancements in gut microbiota (GM) research have shed light on the intricate relationship between GM and TB, suggesting that GM alterations may influence host susceptibility, disease progression, and response to antituberculosis drugs. This review systematically synthesizes and analyzes the current research progress on the relationship between GM and TB, focusing on six key aspects: (1) bidirectional effects between GM dynamics and TB progression; (2) the interaction between GM and anti-TB drugs; (3) GM and TB immune response; (4) GM as a potential target for diagnosis and treatment of TB; (5) multi-omics and artificial intelligence (AI) technologies in GM-TB research; (6) current challenges and future directions in GM-TB research. We highlight the bidirectional nature of the GM-TB interaction, where MTB infection can lead to GM dysbiosis, and changes can affect the host's immune response, contributing to TB onset and progression. Advanced molecular techniques, such as next-generation sequencing and metagenomics, along with AI, play pivotal roles in elucidating these complex interactions. Future research directions include investigating the relationship between GM and TB vaccine efficacy, exploring GM's potential in TB prevention, developing microbiome-based diagnostic and prognostic tools, and examining the role of GM in TB recurrence. By addressing these areas, we aim to provide a comprehensive perspective on the latest advancements in GM and TB research and offer insights for future studies and clinical applications. Ultimately, the development of novel microbiome-based strategies may offer new tools and insights for the effective control and management of TB, a disease that continues to pose a significant threat to public health.
Keywords: Mycobacterium tuberculosis; artificial intelligence; gut microbiota; microbiome‐based diagnostics; omics technologies; tuberculosis.
© 2025 The Author(s). iMeta published by John Wiley & Sons Australia, Ltd on behalf of iMeta Science.
Conflict of interest statement
The authors have declared no competing interests.
Figures












References
-
- World Health Organization. 2024. “Global Tuberculosis Report 2024.” Geneva: World Health Organization: 1−68. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa...
-
- Lin, Zixun , Sun Liqin, Wang Cheng, Wang Fuxiang, Wang Jun, Li Qian, and Lu Hongzhou. 2023. “Bottlenecks and Recent Advancements in Detecting Mycobacterium Tuberculosis in Patients With HIV.” iLABMED 1: 44–57. 10.1002/ila2.11 - DOI
-
- Chen, Zhi , Wang Tao, Du Jingli, Sun Lin, Wang Guirong, Ni Ruizi, An Yajing, et al. 2025. “Decoding the WHO Global Tuberculosis Report 2024: A Critical Analysis of Global and Chinese Key Data.” Zoonoses 5(1): 1. 10.15212/zoonoses-2024-0061 - DOI
Publication types
LinkOut - more resources
Full Text Sources
