Interaction of high pressure-stressed soy protein isolate and 5-methyltetrahydrofolate and its impact on the stability of 5-methyltetrahydrofolate
- PMID: 40860686
- PMCID: PMC12375248
- DOI: 10.1016/j.crfs.2025.101169
Interaction of high pressure-stressed soy protein isolate and 5-methyltetrahydrofolate and its impact on the stability of 5-methyltetrahydrofolate
Abstract
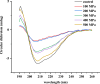
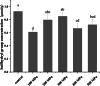
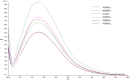
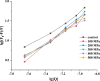
5-Methyltetrahydrofolate (5-MTHF) is considered as a safer fortifier than folic acid (FA), but still unstable and less bioavailable. To investigated the soy protein isolate (SPI) or high hydrostatic pressure (HHP) treated SPI on the thermal stability of 5-MTHF and interaction of protein with 5-MTHF, the thermal stability of 5-MTHF in SPI-5MTHF complexes and HHP treated SPI-5MTHF complex was firstly investigated, and then revealed the effect of HHP on the structure and micromorphology of SPI by circular dichroism spectroscopy, free sulfhydryl content and scanning electron microscopy. Finally, the effect of HHP on the ability of the SPI to bind to 5-MTHF was also investigated by fluorescence spectrometry and isothermal titration. The results showed that the SPI-5MTHF complex was more thermally stable than free 5-MTHF in the thermal environment, and the retention of 5-MTHF was increased by about 306-fold. HHP also improved 5-MTHF stability in the HPP-SPI-5MTHF complex, with the highest retention of 5-MTHF of 104.77 ± 0.22 % at 300MPa. HHP increased apparent binding constants (4.83-26.78-fold) and binding sites (9.22 %-15.60 %) of SPI with 5-MTHF at pressures of 100-300 MPa. The interaction between SPI and 5-MTHF is a spontaneous exothermic reaction driven by enthalpy change. 5-MTHF is mainly bound to SPI by van der Waals forces and hydrogen bonding to form a complex. This study lays the foundation for further application of SPI or HHP-SPI as an encapsulation material to improve the stability and bioaccessibility of 5-MTHF.
Keywords: 5-Methyltetrahydrofolate; High hydrostatic pressure; Interaction; Soy protein isolate; Stability.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Agyenim-Boateng K.G., Zhang S., Shohag M.J.I., Shaibu A.S., Li J., Li B., Sun J. Folate biofortification in soybean: challenges and prospects. Agronomy. 2023;13(1):241. doi: 10.3390/agronomy13010241. - DOI
-
- Ariyarathna I.R., Karunaratne D.N. Use of chickpea protein for encapsulation of folate to enhance nutritional potency and stability. Food Bioprod. Process. 2015;95:76–82. doi: 10.1016/j.fbp.2015.04.004. - DOI
-
- Bationo F., Humblot C., Songré-Ouattara L.T., Hama-Ba F., Le Merrer M., Chapron M., et al. Total folate in West African cereal-based fermented foods: bioaccessibility and influence of processing. J. Food Compos. Anal. 2020;85 doi: 10.1016/j.jfca.2019.103309. - DOI
LinkOut - more resources
Full Text Sources

