Sex-specific risk signals and onset patterns of drug-induced peripheral neuralgia: a 20-year pharmacovigilance analysis based on FAERS real-world dat
- PMID: 40860891
- PMCID: PMC12370653
- DOI: 10.3389/fphar.2025.1628362
Sex-specific risk signals and onset patterns of drug-induced peripheral neuralgia: a 20-year pharmacovigilance analysis based on FAERS real-world dat
Abstract
Background: Peripheral neuralgia is a chronic pain syndrome resulting from peripheral nerve damage and has been increasingly linked to certain drugs, leading to drug-induced peripheral neuropathy (DIPN). While the neurotoxic potential of many drugs has been recognized, the gender-specific patterns of DIPN remain insufficiently studied.
Objective: To identify potential drug safety signals associated with DIPN and explore gender-based differences in risk using real-world pharmacovigilance data.
Methods: This retrospective pharmacovigilance study utilized the FDA Adverse Event Reporting System (FAERS) database from 2004 to 2024. Disproportionality analysis (DPA), specifically Reporting Odds Ratio (ROR), was applied to detect associations between drugs and DIPN. Drug and adverse event terms were standardized using RxNorm and MedDRA dictionaries. Weibull distribution modeling was employed to analyze time-to-onset (TTO) characteristics of high-risk drugs in male and female populations.
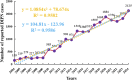
Results: A total of 21,609 adverse event reports of DIPN were analyzed, showing a continuous increase in reporting over two decades. Seventy-two drugs were identified as having potential DIPN risk signals, with 25 drugs showing strong associations after statistical adjustments. Among them, adalimumab, ciprofloxacin, and lenalidomide had the highest number of reports. Eighteen drugs presented new risk signals not previously mentioned in official drug labeling. Gender-specific analysis revealed 49 risk drugs in females, 32 in males, with 23 drugs overlapping. Time-to-onset analysis showed most adverse events occurred early in treatment, as indicated by Weibull shape parameters (β < 1) for all major drugs.
Conclusion: This study revealed novel and sex-specific DIPN risk signals using large-scale real-world data. It highlights the importance of early monitoring of neurotoxic effects during drug treatment and provides strong support for implementing gender-sensitive pharmacovigilance strategies and individualized medication risk management.
Keywords: FAERS; disproportionality analysis; drug-induced peripheral neuropathy (DIPN); gender differences; pharmacovigilance.
Copyright © 2025 Wang, Deng, Liao, Quan and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Drug-Related epileptic seizures and Age-Specific Differences: A Real-World study based on the FAERS database.Epilepsy Behav. 2025 Jul 31;171:110638. doi: 10.1016/j.yebeh.2025.110638. Online ahead of print. Epilepsy Behav. 2025. PMID: 40749454
-
Sex differences in drug-induced osteoporosis: a pharmacovigilance study based on the FAERS database.Front Public Health. 2025 Jul 24;13:1630412. doi: 10.3389/fpubh.2025.1630412. eCollection 2025. Front Public Health. 2025. PMID: 40777658 Free PMC article.
-
Adverse drug reactions related to methotrexate: a real-world pharmacovigilance study using the FAERS database from 2004 to 2024.Front Immunol. 2025 Jun 4;16:1586361. doi: 10.3389/fimmu.2025.1586361. eCollection 2025. Front Immunol. 2025. PMID: 40534848 Free PMC article. Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Bennett A. C., Bennett C. L., Witherspoon B. J., Knopf K. B. (2019). An evaluation of reports of ciprofloxacin, levofloxacin, and moxifloxacin-association neuropsychiatric toxicities, long-term disability, and aortic aneurysms/dissections disseminated by the food and drug administration and the european medicines agency. Expert Opin. Drug Saf. 18 (11), 1055–1063. 10.1080/14740338.2019.1665022 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

