Vonoprazan-minocycline dual therapy as a first-line treatment of Helicobacter pylori infection compared with empirical bismuth-containing quadruple therapy
- PMID: 40860932
- PMCID: PMC12374092
- DOI: 10.1177/17562848251366156
Vonoprazan-minocycline dual therapy as a first-line treatment of Helicobacter pylori infection compared with empirical bismuth-containing quadruple therapy
Abstract
Background: Increasing antibiotic resistance compromises therapeutic options for Helicobacter pylori (H. pylori) infection, especially in penicillin-allergic individuals.
Objectives: This trial aimed to assess the efficacy and safety of 14-day vonoprazan-minocycline (VM) dual therapy against bismuth-containing quadruple therapy (B-quadruple therapy), as initial treatment for H. pylori infection.
Design: This study was a single-center, open-label, and non-inferiority randomized controlled trial.
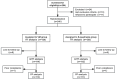
Methods: In this study, 240 individuals with H. pylori infection who have not received therapy were randomly assigned 1:1 to either the VM dual therapy group (vonoprazan 20 mg plus minocycline 100 mg, administered twice daily) or the B-quadruple therapy group (rabeprazole 10 mg, amoxicillin 1000 mg, clarithromycin 500 mg, and bismuth potassium citrate 220 mg, all administered twice daily). The primary outcome was to evaluate the non-inferiority of eradication rates between the two groups. Secondary outcomes included assessments of AEs and compliance.
Results: The eradication rates of VM dual group and B-quadruple therapy group were 87.5% and 88.3%, respectively, by intention-to-treat (ITT) analysis; 92.1% and 94.6% by modified ITT (mITT) analysis; and 92.0% and 95.5% by per-protocol (PP) analysis. The eradication rates of the VM group were non-inferior to those of the B-quadruple therapy group in ITT, mITT, and PP analyses (one-sided p-values were 0.02, 0.01, and 0.02). The incidence of AEs was higher in the B-quadruple therapy group (28.3%) than in the VM group (16.7%, p = 0.03). Good compliance was achieved in both groups (p = 0.60).
Conclusion: The VM dual therapy was not inferior to the B-quadruple therapy in the initial treatment of H. pylori infection, and the incidence of AEs was lower compared to B-quadruple therapy.
Trial registration: This trial was registered on the Chinese Clinical Trial Registry with the registration number ChiCTR2400081461.
Keywords: Helicobacter pylori; bismuth-containing quadruple therapy; dual therapy; minocycline.
Plain language summary
Fourteen-day vonoprazan-minocycline dual therapy versus bismuth-containing quadruple therapy for first-line helicobacter pylori eradication: a single-center randomized clinical trial Increasing antibiotic resistance compromises therapeutic options for Helicobacter pylori (H. pylori) infection, especially in penicillin-allergic individuals. This trial aimed to assess the efficacy and safety of 14-day vonoprazan-minocycline (VM) dual therapy against bismuth-containing quadruple therapy (B-quadruple therapy), as initial treatment for H. pylori infection. The VM dual therapy was not inferior to B-quadruple therapy in the initial treatment of H. pylori infection, and the incidence of AEs was lower compared to B-quadruple therapy.
© The Author(s), 2025.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
References
-
- Malfertheiner P, Megraud F, Rokkas T, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut 2022; 71: 1724–1762.
-
- Yu Y, Xue J, Lin F, et al. Global primary antibiotic resistance rate of Helicobacter pylori in recent 10 years: a systematic review and meta-analysis. Helicobacter 2024; 29(3): e13103. - PubMed
-
- Chey WD, Howden CW, Moss SF, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol 2024; 119: 1730–1753. - PubMed
-
- Song Z, Chen Y, Lu H, et al. Diagnosis and treatment of Helicobacter pylori infection by physicians in China: a nationwide cross-sectional study. Helicobacter 2022; 27(3): e12889. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous