Reduced Platelet Aggregation and Plasma Cytokine Levels Mitigate Progressive Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
- PMID: 40860949
- PMCID: PMC12375347
- DOI: 10.2147/JIR.S533707
Reduced Platelet Aggregation and Plasma Cytokine Levels Mitigate Progressive Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
Abstract
Purpose: Patients with metabolic syndrome and coronary artery disease (CAD) are at increased risk of metabolic dysfunction-associated steatotic liver disease (MASLD), which can progress to steatohepatitis, cirrhosis, and hepatocellular carcinoma. MASLD is the most common liver disease and a significant contributor to cardiovascular morbidity. Enhanced platelet aggregation is linked to steatohepatitis, and antiplatelet therapy has been suggested as a potential treatment.
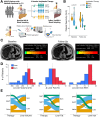
Patients and methods: In a prospective study of 51 patients with type 2 diabetes mellitus and/or obesity (BMI≥30), we evaluated the impact of antiplatelet therapy on hepatic fat content, liver volume, and iron deposition using magnetic resonance imaging (MRI) at baseline and six months. Ex vivo platelet function testing and plasma levels of proinflammatory chemotactic cytokines were measured to characterize thromboinflammatory mechanisms underlying MASLD.
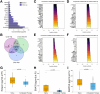
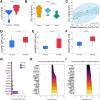
Results: Increased platelet reactivity correlated with greater hepatic fat, iron deposition, and liver volume. Antiplatelet therapy was associated with reductions in hepatic volume and iron accumulation. Progression of steatosis was linked to dyslipidemia, platelet hyperreactivity, and elevated plasma levels of profibrotic, inflammatory, and apoptotic chemokines/cytokines. A distinct systemic cytokine profile corresponded with morphological features of progressive MASLD.
Conclusion: Reduced platelet aggregation is associated with attenuation of MASLD features. Antiplatelet therapy correlates with decreased pro-inflammatory and pro-fibrotic chemokine signaling linked to the morphological characteristics of MASLD. Assessment of platelet reactivity and specific chemokines may enhance understanding of MASLD pathophysiology and support the development of novel therapeutic strategies.
Keywords: antiplatelet treatment; chemokine signaling; coronary artery disease; steatosis.
© 2025 Harm et al.
Conflict of interest statement
Dr Michal Droppa reports personal fees from AstraZeneca, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures


Similar articles
-
Metabolic Dysfunction-Associated Steatotic Liver Disease (MΑSLD).2025 Aug 9. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Aug 9. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31082077 Free Books & Documents.
-
The Significance of the Presence of Gilbert's Syndrome in Patients With Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A Retrospective Cohort Study.Cureus. 2025 May 30;17(5):e85074. doi: 10.7759/cureus.85074. eCollection 2025 May. Cureus. 2025. PMID: 40585738 Free PMC article.
-
Platelet Functional Profile Is Altered in Metabolic Dysfunction-Associated Steatotic Liver Disease.Liver Int. 2025 Aug;45(8):e70231. doi: 10.1111/liv.70231. Liver Int. 2025. PMID: 40719417 Free PMC article.
-
Metabolic-dysfunction associated steatotic liver disease and atrial fibrillation: A review of pathogenesis.World J Cardiol. 2025 Jun 26;17(6):106147. doi: 10.4330/wjc.v17.i6.106147. World J Cardiol. 2025. PMID: 40575425 Free PMC article. Review.
-
Silymarin for adults with metabolic dysfunction-associated steatotic liver disease.Cochrane Database Syst Rev. 2025 Jun 24;6(6):CD015524. doi: 10.1002/14651858.CD015524.pub2. Cochrane Database Syst Rev. 2025. PMID: 40552569
References
LinkOut - more resources
Full Text Sources
Miscellaneous

