Costs of First-Line Treatment With FOLFIRINOX, Modified FOLFIRINOX, and Gemcitabine With Nab-Paclitaxel in Metastatic Pancreatic Ductal Adenocarcinoma
- PMID: 40861017
- PMCID: PMC12375408
- DOI: 10.36469/001c.142403
Costs of First-Line Treatment With FOLFIRINOX, Modified FOLFIRINOX, and Gemcitabine With Nab-Paclitaxel in Metastatic Pancreatic Ductal Adenocarcinoma
Abstract
Background: Further research is needed to determine real-world costs of first-line (1L) treatment of metastatic pancreatic ductal adenocarcinoma (mPDAC) with FOLFIRINOX (FFX), modified FFX (mFFX), and gemcitabine with nab-paclitaxel (GnP).
Objectives: To describe healthcare costs by treatment regimen, stratified by commercial and Medicare Advantage insurance.
Methods: This retrospective cohort study of adult patients with mPDAC utilized Optum's de-identified Market Clarity Dataset. Demographics, clinical characteristics, and 1L unadjusted all-cause healthcare costs were examined. Total all-cause costs included costs from inpatient, outpatient, chemotherapy drug and administration, granulocyte colony-stimulating factor (G-CSF), radiation therapy, and other outpatient and pharmacy costs.
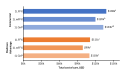
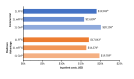
Results: A total of 3115 patients met the criteria for inclusion and received 1L treatment with either FFX, mFFX, or GnP. Among those, 1703 had commercial insurance (FFX, 536; mFFX, 673; GnP, 494) and 1412 had Medicare Advantage (FFX, 201; mFFX, 317; GnP, 894). Total cost of care (mean [SD]) was similar between regimens for each insurance cohort (mean [SD] commercial: FFX, 127 504]; mFFX, 112 208]; GnP, 154 248]; Medicare Advantage: FFX, 98 492]; mFFX, 83 437]; GnP, 100 150]). For both insurance cohorts, chemotherapy drug costs were highest for GnP (mean [SD] commercial: FFX, 21 647]; mFFX, 10 054]; GnP, 112 589]; Medicare Advantage: FFX, 11 044]; mFFX, 7688]; GnP, 49 373]), while chemotherapy administration costs were higher for FFX and mFFX (commercial: FFX, 33 350]; mFFX, 24 309]; GnP 15 766]; Medicare Advantage: FFX, 36 352]; mFFX, 22 317]; GnP 13 089]). G-CSF costs were also higher for FFX and mFFX (commercial: FFX, 56 593], mFFX, 41 166]; GnP, 14 181]; Medicare Advantage: FFX, 56 630]; mFFX, 39 286]; GnP, 9115]).
Discussion: Total costs of 1L FFX, mFFX, and GnP were similar within a commercially insured and Medicare Advantage cohort. FFX and mFFX costs were largely driven by chemotherapy administration and G-CSF costs, while GnP costs were driven by chemotherapy drug costs.
Conclusions: To fully assess the economic impact of mPDAC in 1L treatment, it is essential to consider both the total cost and the individual cost components, such as chemotherapy drugs, administration, and supportive care costs.
Keywords: cost drivers; healthcare resource utilization; pancreatic cancer; payer perspectives; real-world evidence; supportive care.
Conflict of interest statement
S.D., M.M., and E.N. are employees of Genesis Research Group, which received consulting fees from Ipsen Biopharmaceuticals Inc. P.C. reports former employment and equity holder in Ipsen Biopharmaceuticals, Inc. R.P. has received honorarium for consultation and speaking from Ipsen Biopharmaceuticals Inc. and Seagen and for consultation from Exelixis.
Figures


References
-
- National Cancer Institute, Surveillance Epidemiology and End Results Program Cancer Stat Facts: Pancreatic Cancer. [2024-9-26]. https://seer.cancer.gov/statfacts/html/pancreas.html
-
- Cancer statistics, 2024. Siegel R. L., Giaquinto A. N., Jemal A. 2024CA Cancer J Clin. 74(1):12–49. doi: 10.3322/caac.21820. https://doi.org/10.3322/caac.21820 - DOI - PubMed
-
- Pancreatic cancer: advances and challenges. Halbrook C. J., Lyssiotis C. A., Pasca di Magliano M., Maitra A. 2023Cell. 186(8):1729–1754. doi: 10.1016/j.cell.2023.02.014. https://doi.org/10.1016/j.cell.2023.02.014 - DOI - PMC - PubMed
-
- Real-world prognostic factors for survival among treated patients with metastatic pancreatic ductal adenocarcinoma. Yu K. H., Ozer M., Cockrum P., Surinach A., Wang S., Chu B. C. 2021Cancer Med. 10(24):8934–8943. doi: 10.1002/cam4.4415. https://doi.org/10.1002/cam4.4415 - DOI - PMC - PubMed
-
- Treatment costs and social burden of pancreatic cancer. Cipora E., Partyka O., Pajewska M.., et al. 2023Cancers (Basel) 15(6):1911. doi: 10.3390/cancers15061911. https://doi.org/10.3390/cancers15061911 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
