Thoracic paravertebral osteosarcoma induced by radiotherapy for esophageal cancer: A case report
- PMID: 40861103
- PMCID: PMC12373421
- DOI: 10.3892/ol.2025.15231
Thoracic paravertebral osteosarcoma induced by radiotherapy for esophageal cancer: A case report
Abstract
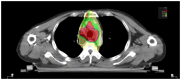
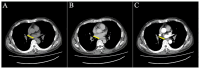
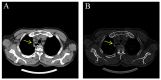
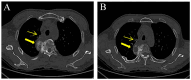
With the wide application of radiation therapy for malignant tumors and the continuous improvement of comprehensive treatment effect, the survival period of patients has been prolonged, while the incidence of radiation-induced osteosarcoma (RIOS) has gradually increased. Compared with primary osteosarcoma, RIOS has a higher degree of malignancy and poorer prognosis, severely impacting patient survival. Currently, there are relatively few case reports on RIOS and the understanding of its imaging characteristics remains incomplete. A patient with esophageal cancer who was found to have thoracic paravertebral masses six years after receiving radiotherapy was encountered at Zhuji People's Hospital (Zhuji, China). Computed tomography (CT), magnetic resonance imaging and positron emission tomography/CT all indicated the presence of the malignant tumors. Subsequently, the patient was hospitalized for CT-guided puncture biopsy of thoracic paravertebral masses. Through multidisciplinary discussions in the departments of Medical Oncology, Orthopedics, Radiology and Pathology, a consensus was finally reached on RIOS. In conclusion, RIOS is a severe and relatively rare complication of radiotherapy with a poor prognosis. In its early stage, it is easily confused with bone changes after radiotherapy and appearance deformities after surgery. Neoplastic bone is the primary imaging feature of RIOS of esophageal cancer. By combining the patient's radiotherapy history and laboratory examinations, the diagnostic accuracy for this disease could be improved.
Keywords: CT; MRI; PET/CT; radiation-induced osteosarcoma; radiotherapy.
Copyright: © 2025 Chen et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
123I-MIBG scintigraphy and 18F-FDG-PET imaging for diagnosing neuroblastoma.Cochrane Database Syst Rev. 2015 Sep 29;2015(9):CD009263. doi: 10.1002/14651858.CD009263.pub2. Cochrane Database Syst Rev. 2015. PMID: 26417712 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Anterior Approach Total Ankle Arthroplasty with Patient-Specific Cut Guides.JBJS Essent Surg Tech. 2025 Aug 15;15(3):e23.00027. doi: 10.2106/JBJS.ST.23.00027. eCollection 2025 Jul-Sep. JBJS Essent Surg Tech. 2025. PMID: 40821726 Free PMC article.
-
Positron emission tomography-adapted therapy for first-line treatment in individuals with Hodgkin lymphoma.Cochrane Database Syst Rev. 2015 Jan 9;1(1):CD010533. doi: 10.1002/14651858.CD010533.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2025 Mar 26;3:CD010533. doi: 10.1002/14651858.CD010533.pub3. PMID: 25572491 Free PMC article. Updated.
References
Publication types
LinkOut - more resources
Full Text Sources
