Exercise in atherosclerosis: its beneficial effects and underlying mechanism
- PMID: 40861271
- PMCID: PMC12375675
- DOI: 10.3389/fcell.2025.1598794
Exercise in atherosclerosis: its beneficial effects and underlying mechanism
Abstract
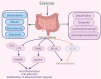
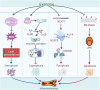
Atherosclerosis represents a complex interplay of inflammatory and metabolic processes, in which oxidative stress, endothelial inflammation, the phenotypic transition of smooth muscle cells (SMCs), and the conversion of macrophages into foam cells are involved. In contrast to pharmacological interventions, exercise emerges as a viable, cost-effective, and low-risk strategy to alleviate the progression of atherosclerosis. Exercise exerts beneficial effects on atherosclerosis through modulation of diverse pathways, including exerkines, browning of adipose tissue, the renin-angiotensin system (RAS), metabolites, gut microbiota, cell death pathways, microRNAs, nervous system, and immune function. The beneficial impacts of exercise on atherosclerosis and the mechanisms behind them will be examined here. Fully understanding the effects and mechanisms of exercise in reducing atherosclerosis might open doors to developing safe and effective interventions.
Keywords: atherosclerosis; browning of adipose tissue; cell death; exercise; exerkines; immune function; microRNAs.
Copyright © 2025 Yin and Guo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources

