Ferroptosis and gastric cancer: from molecular mechanisms to clinical implications
- PMID: 40861444
- PMCID: PMC12375570
- DOI: 10.3389/fimmu.2025.1581928
Ferroptosis and gastric cancer: from molecular mechanisms to clinical implications
Abstract
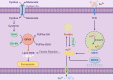
Gastric cancer, one of the leading causes of cancer-related mortality globally, faces challenges in treatment due to limitations in surgery, chemotherapy resistance, and high recurrence rates. Ferroptosis, an iron-dependent form of cell death, induces cell membrane rupture through dysregulated iron metabolism, lipid peroxidation, and the accumulation of reactive oxygen species (ROS), offering a promising therapeutic avenue for gastric cancer treatment. This article systematically explores the core mechanisms of ferroptosis, including iron overload catalyzing lipid peroxidation via the Fenton reaction, dysregulation of antioxidant systems (such as GPX4 and FSP1), and their associations with gastric cancer cell proliferation, metastasis, and resistance. Studies indicate that abnormalities in iron metabolism in gastric cancer cells, such as upregulation of TFR1 and dysregulated ferritin storage, significantly promote ferroptosis sensitivity, while ferroptosis inducers (such as Erastin and RSL3) can enhance chemotherapy sensitivity and reverse resistance by inhibiting GPX4 or system Xc-. Preclinical experiments confirm that targeting ferroptosis-related pathways (such as the USP7/SCD axis and ABCC2-mediated glutathione efflux) effectively inhibits tumor growth and metastasis. However, the dual-edged effect of ferroptosis warrants caution regarding its oxidative damage risk to normal tissues and potential pro-metastatic mechanisms. This article further proposes the potential of ferroptosis biomarkers (such as 4-HNE and GPX4) in early diagnosis and prognosis assessment of gastric cancer and emphasizes the need for precision medicine to optimize ferroptosis-targeted strategies, balancing efficacy and safety. Ferroptosis opens a new avenue for gastric cancer treatment, but its clinical translation still requires in-depth mechanistic exploration and personalized treatment plan design.
Keywords: GPx4; ferroptosis; gastric cancer; lipid peroxidation; treatment resistance.
Copyright © 2025 Zhao, Ao, Sorina, Wei, Yin, Zhang, Lee, Du and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Dioscin induces ferroptosis to suppress the metastasis of gastric cancer through the SLC7A11/GPX4 axis.Free Radic Res. 2025 May;59(5):426-441. doi: 10.1080/10715762.2025.2515202. Epub 2025 Jun 13. Free Radic Res. 2025. PMID: 40460255
-
Perillaldehyde synergizes with ferroptosis inducers to promote ferroptotic cell death in gastric cancer.Front Cell Dev Biol. 2025 Jun 3;13:1598520. doi: 10.3389/fcell.2025.1598520. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40530331 Free PMC article.
-
Ferroptosis: a potential therapeutic target in cardio-cerebrovascular diseases.Mol Cell Biochem. 2025 Jul;480(7):4379-4399. doi: 10.1007/s11010-025-05262-7. Epub 2025 Mar 27. Mol Cell Biochem. 2025. PMID: 40148662 Review.
-
Targeting ferroptosis using Chinese herbal compounds to treat respiratory diseases.Phytomedicine. 2024 Jul 25;130:155738. doi: 10.1016/j.phymed.2024.155738. Epub 2024 Jun 1. Phytomedicine. 2024. PMID: 38824825
-
African swine fever virus MGF505-3R facilitates ferroptosis to restrict TBK1-IRF3 pathway.Microbiol Spectr. 2025 Aug 5;13(8):e0342324. doi: 10.1128/spectrum.03423-24. Epub 2025 Jun 23. Microbiol Spectr. 2025. PMID: 40548711 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

