A Comparative Assessment of Intraocular Pressure Changes After Aflibercept 8 mg and Faricimab-svoa Intravitreal Injections in Wet Age-Related Macular Degeneration
- PMID: 40861593
- PMCID: PMC12373418
- DOI: 10.7759/cureus.88617
A Comparative Assessment of Intraocular Pressure Changes After Aflibercept 8 mg and Faricimab-svoa Intravitreal Injections in Wet Age-Related Macular Degeneration
Abstract
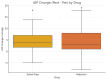
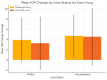
Introduction A standard practice for addressing wet age-related macular degeneration (WetAMD) is to deliver anti-vascular endothelial growth factor (anti-VEGF) intravitreal injections. Formulations with higher concentrations, such as aflibercept 8 mg (Eylea HD; Bayer AG, Leverkusen, Germany), which are administered in larger volumes, may raise concerns about potential increases in intraocular pressure (IOP) and other ocular complications. The objective of this study was to evaluate and compare short-term IOP changes following intravitreal injection of aflibercept 8 mg (Eylea HD, 0.07 mL) versus faricimab-svoa (Vabysmo, 0.05 mL; Roche Pharma, Basel, Switzerland/Genentech, South San Francisco, CA, USA) in patients with WetAMD. Methods A retrospective observational analysis was conducted involving patients with WetAMD who received intravitreal injections of aflibercept 8 mg (n = 64 eyes) or faricimab-svoa (n = 73 eyes). IOP was measured before the injection and 30 minutes after. The research recorded lens condition, the need for paracentesis, and the application of Iopidine drops. Patients were categorized into phakic and pseudophakic subgroups, and further stratified based on post-injection IOP levels: <20 mmHg, 20-25 mmHg, 25-30 mmHg, and >30 mmHg. Results Both treatment groups showed notable increases in IOP at 30 minutes after injection (Eylea HD: +4.50 ± 4.32 mmHg, Vabysmo: +3.66 ± 5.20 mmHg; p = 0.083). However, there were no instances requiring paracentesis, and only one patient from each group needed Iopidine drops. Pseudophakic patients experienced slightly higher IOP increases (Eylea HD: +4.71 ± 4.18 mmHg, Vabysmo: +4.55 ± 5.65 mmHg; p = 0.784) compared to phakic patients. The majority of patients maintained IOP levels under 30 mmHg. Gender distribution was 49% male and 51% female. Conclusions Intravitreal injections of aflibercept 8 mg and faricimab-svoa caused a small and temporary increase in IOP and there were no cases requiring urgent management. Our results confirm the short-term ocular safety of aflibercept 8 mg and faricimab-svoa for the treatment of WetAMD and highlight the need for individualized monitoring for patients at risk of increased IOP.
Keywords: aflibercept 8mg intravitreal injection; anti-vegf treatment; faricimab intravitreal injection; intraocular pressure (iop); wet-aged related macular degeneration.
Copyright © 2025, Gartaganis et al.
Conflict of interest statement
Human subjects: Informed consent for treatment and open access publication was obtained or waived by all participants in this study. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
LinkOut - more resources
Full Text Sources
