High-performance corrosion inhibitors for carbon steel in hydrochloric acid: electrochemical and DFT studies
- PMID: 40861989
- PMCID: PMC12377231
- DOI: 10.1039/d5ra04952k
High-performance corrosion inhibitors for carbon steel in hydrochloric acid: electrochemical and DFT studies
Abstract
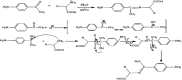
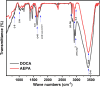
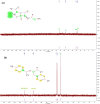
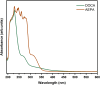
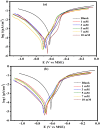
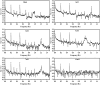
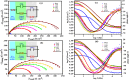
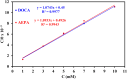
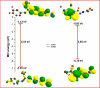
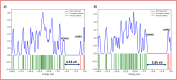
This research examines the corrosion inhibition efficiency of two novel compounds, AEPA and DOCA, on carbon steel in 1.0 M hydrochloric acid. Both AEPA and DOCA demonstrated excellent electrochemical performance as corrosion inhibitors, with inhibition efficiencies exceeding 93% at a concentration of 10 mM, as confirmed through potentiodynamic polarization (PDP), electrochemical frequency modulation (EFM), and electrochemical impedance spectroscopy (EIS) techniques. EIS analysis revealed a marked increase in charge transfer resistance (R ct), reaching 387.55 Ω cm2 for AEPA and 345.80 Ω cm2 for DOCA, indicating the formation of a robust protective layer on the carbon steel surface. Adsorption studies confirmed that both inhibitors follow the Langmuir isotherm model, suggesting monolayer chemisorption. The calculated adsorption equilibrium constants with corresponding Gibbs free energy values of -29.53 kJ per mol (AEPA) and -29.30 kJ per mol (DOCA), respectively, indicating spontaneous and strong adsorption interactions. Theoretical insights from density functional theory (DFT) calculations revealed that AEPA possesses a higher HOMO energy (-5.65 eV) and a lower LUMO energy (-1.12 eV) compared to DOCA (HOMO: -6.70 eV, LUMO: -0.85 eV), resulting in a smaller energy gap (ΔE = 4.53 eV for AEPAvs. 5.85 eV for DOCA). This suggests that AEPA has a greater electron-donating ability and stronger interaction with the metal surface. The integration of experimental and theoretical approaches provides a comprehensive understanding of their inhibition mechanisms and highlights their potential for practical applications in corrosion protection.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Modwi M. M. Y. Feng H. Hadi M. K. Chen N. Hou J. Kamal E. Yang K. Eco-friendly corrosion inhibitor of Q235 carbon steel in 1.0 M HCl by Isatin/Chitosan Schiff base. J. Mol. Struct. 2025;1321:139592. doi: 10.1016/j.molstruc.2024.139592. - DOI
-
- Kaya F. Solmaz R. Geçibesler İ. H. Adsorption and corrosion inhibition capability of Rheum ribes root extract (Işgın) for mild steel protection in acidic medium: A comprehensive electrochemical, surface characterization, synergistic inhibition effect, and stability study. J. Mol. Liq. 2023;372:121219. doi: 10.1016/j.molliq.2023.121219. - DOI
-
- Yousif Q. A. Ranjeh M. Ghiyasiyan-Arani M. Al-Nayili A. Monsef R. Salavati-Niasari M. Morphology engineering of LiFeO2 nanostructures through synthesis controlling for electrochemical hydrogen storage inquiries. Fuel. 2022;313:123025. doi: 10.1016/j.fuel.2021.123025. - DOI
-
- Desai P. D. Pawar C. B. Avhad M. S. More A. P. Corrosion inhibitors for carbon steel: A review. Vietnam J. Chem. 2023;61:15–42. doi: 10.1002/vjch.202200111. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

