Chimeric Antigen Receptor T Cell Immunotherapy for Autoimmune Rheumatic Disorders: Where Are We Now?
- PMID: 40862721
- PMCID: PMC12384554
- DOI: 10.3390/cells14161242
Chimeric Antigen Receptor T Cell Immunotherapy for Autoimmune Rheumatic Disorders: Where Are We Now?
Abstract
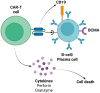
Chimeric antigen receptor (CAR) T cell immunotherapy has changed the landscape of B cell hematological malignancies' management, while it has recently shown promising results in the treatment of refractory autoimmune rheumatic disorders (ARDs). Targeting B cell antigens such as CD19 and BCMA, CAR-T cell therapy can induce sustained remission by the elimination of autoreactive B cell populations resistant to the standard of care treatment options. Clinical data from case reports and small case series demonstrate profound clinical responses in ARDs, including systemic lupus erythematosus (SLE), systemic sclerosis (SSc), idiopathic inflammatory myopathies (IIMs), rheumatoid arthritis (RA), antiphospholipid syndrome (APS), and primary Sjögren's syndrome (pSS). Treatment outcomes include reduced disease activity, normalization of serologic markers, improved organ function, and drug-free remission, even after B cell reconstitution. Additionally, toxicities, primarily limited to mild cytokine release syndrome (CRS), were generally manageable with supportive care. Encouraging preliminary results have led to the development of several ongoing clinical trials investigating CAR-T cell therapy across multiple ARDs and patient populations, including pediatric patients. This review summarizes the current clinical experience and provides a comprehensive overview of ongoing clinical trials exploring CAR-T cell immunotherapy for ARDs.
Keywords: BCMA; CAR-T cell therapy; CD19; autoimmune rheumatic disorders; immunotherapy; systemic lupus erythematosus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

