Post-Translational Modifications in Mammalian Folliculogenesis and Ovarian Pathologies
- PMID: 40862771
- PMCID: PMC12385027
- DOI: 10.3390/cells14161292
Post-Translational Modifications in Mammalian Folliculogenesis and Ovarian Pathologies
Abstract
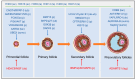
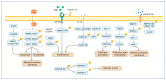
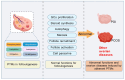
Post-translational modifications (PTMs) of proteins, as the core mechanism for dynamically regulating follicular development, affect the maintenance of mammalian fertility by precisely coordinating granulosa cell-oocyte interaction, metabolic reprogramming, and epigenetic remodeling. Dysregulation of these modifications directly contributes to major reproductive diseases, including polycystic ovary syndrome (PCOS) and premature ovarian insufficiency (POI). Post-translational modifications regulate follicular development through intricate mechanisms. Thus, this review systematically synthesizes recent advances in PTMs, encompassing traditional ones such as phosphorylation, ubiquitination, and acetylation, alongside emerging modifications including lactylation, SUMOylation, and ISGylation, thereby constructing a more comprehensive PTM landscape of follicular development. Furthermore, this study dissects the molecular interaction networks of these PTMs during follicular activation, maturation, and ovulation, and uncovers the common mechanisms through which PTM dysregulation contributes to pathological conditions, including hyperandrogenism in PCOS and follicular depletion in POI. Finally, this review ultimately provides a theoretical basis for improving livestock reproductive efficiency and precise intervention in clinical ovarian diseases.
Keywords: follicular development; polycystic ovary syndrome; post-translational modification; premature ovarian insufficiency.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Nguyen X.P., Vilkaite A., Bender U., Dietrich J.E., Hinderhofer K., Strowitzki T., Rehnitz J. Regulation of Bone Morphogenetic Protein Receptor Type II Expression by FMR1/Fragile X Mental Retardation Protein in Human Granulosa Cells in the Context of Poor Ovarian Response. Int. J. Mol. Sci. 2024;25:10643. doi: 10.3390/ijms251910643. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

