A New Approach for Interferent-Free Amperometric Biosensor Production Based on All-Electrochemically Assisted Procedures
- PMID: 40862931
- PMCID: PMC12384068
- DOI: 10.3390/bios15080470
A New Approach for Interferent-Free Amperometric Biosensor Production Based on All-Electrochemically Assisted Procedures
Abstract
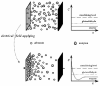
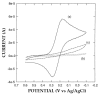
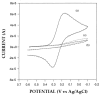
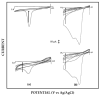
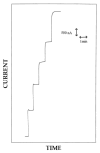
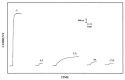
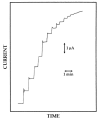
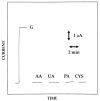
A new approach in amperometric enzyme electrodes production based on all-electrochemically assisted procedures will be described. Enzyme (glucose oxidase) immobilization was performed by in situ co-crosslinking of enzyme molecules through electrophoretic protein deposition, assuring enzyme immobilization exclusively onto the transducer surface (Pt electrode). Analogously, the poor selectivity of the transducer was dramatically improved by the electrosynthesis of non-conducting polymers with built-in permselectivity, permitting the formation of a thin permselective film onto the transducer surface, able to reject common interferents usually found in real samples. Since both approaches required a proper and distinct electrochemical perturbation (a pulsed current sequence for electrophoretic protein deposition and cyclic voltammetry for the electrosynthesis of non-conducting polymers), an appropriate coupling of the two all-electrochemical approaches was assured by a thorough study of the likely combinations of the electrosynthesis of permselective polymers with enzyme immobilization by electrophoretic protein deposition and by the use of several electrosynthesized polymers. For each investigated combination and for each polymer, the analytical performances and the rejection capabilities of the resulting biosensor were acquired so to gain information about their sensing abilities eventually in real sample analysis. This study shows that the proper coupling of the two all-electrochemical approaches and the appropriate choice of the electrosynthesized, permselective polymer permits the easy fabrication of novel glucose oxidase biosensors with good analytical performance and low bias in glucose measurement from typical interferent in serum. This novel approach, resembling classical electroplating procedures, is expected to allow all the advantages expected from such procedures like an easy preparation biosensor, a bi-dimensional control of enzyme immobilization and thickness, interferent- and fouling-free transduction of the electrodic sensor and, last but not the least, possibility of miniaturization of the biosensing device.
Keywords: biosensor; electroanalytical chemistry; electrophoretic enzyme immobilization; electropolymerization; glucose biosensor; permselective polymers.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Turner A.P.F. Current trends in biosensor research and development. Sens. Actuators. 1989;17:433–450. doi: 10.1016/0250-6874(89)80030-7. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

