Behavior of Osteoblastic Lineage Cells When in the Presence of Tamoxifen: In Vitro and In Vivo Studies on Osseointegration
- PMID: 40863054
- PMCID: PMC12384849
- DOI: 10.3390/dj13080351
Behavior of Osteoblastic Lineage Cells When in the Presence of Tamoxifen: In Vitro and In Vivo Studies on Osseointegration
Abstract
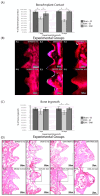
Background/Objectives: Tamoxifen, a selective estrogen receptor modulator widely used as an adjunct in the treatment of breast cancer, has known effects on bone metabolism, although its impact on osseointegration and cellular responses during early bone healing remains unclear. Understanding these effects is essential given the increasing use of dental implants in cancer survivors. The study aimed to observe the influence of tamoxifen on human osteosarcoma (SAOS-2) cells lines, as well on the osseointegration of titanium implants in ovariectomized female rats. Methods: SAOS-2 cells were incubated with Dulbecco's modified growth medium. Six titanium (Ti) disks were used at each time point. The samples were divided into groups with the presence (TAM, n = 36) or not (CTR, n = 36) of tamoxifen in a concentration of 2 μM. In vivo, 72 animals were divided in groups with bilateral ovariectomy or SHAM and tamoxifen administration or not (15 mg/kg). Cell viability, mineralization rate, and collagen synthesis were assessed, as well as bone/implant contact (BIC) and bone ingrowth (BIN). Results: Tamoxifen caused a decrease in SAOS-2 viability, although an increase in the mineralization rate was observed. In vivo, the TAM groups presented higher BIC and BIN when compared to their control, but a lower percentage of mature collagen cells. Conclusions: Based on our findings, in vitro, the therapy with TAM slightly reduced the viability of SAOS-2 cells while significantly increasing the mineralization rate. In vivo, the therapy positively influenced BIC and BIN during the osseointegration phase.
Keywords: cell culture; selective estrogen receptor modulators; tamoxifen; titanium implants.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
The safety and efficacy of controlled ovarian hyperstimulation for fertility preservation in women with early breast cancer: a systematic review.Hum Reprod. 2017 May 1;32(5):1033-1045. doi: 10.1093/humrep/dex027. Hum Reprod. 2017. PMID: 28333356
-
Does Augmenting Irradiated Autografts With Free Vascularized Fibula Graft in Patients With Bone Loss From a Malignant Tumor Achieve Union, Function, and Complication Rate Comparably to Patients Without Bone Loss and Augmentation When Reconstructing Intercalary Resections in the Lower Extremity?Clin Orthop Relat Res. 2025 Jun 26;483(9):1680-1695. doi: 10.1097/CORR.0000000000003599. Clin Orthop Relat Res. 2025. PMID: 40569278
-
Does vitamin D have an effect on osseointegration of dental implants? A systematic review.Int J Implant Dent. 2022 Apr 11;8(1):16. doi: 10.1186/s40729-022-00414-6. Int J Implant Dent. 2022. PMID: 35403929 Free PMC article.
-
Fabrication of anodic and atomic layer deposition-alumina coated titanium implants for effective osteointegration applications.J Biomed Mater Res A. 2025 Jan;113(1):e37792. doi: 10.1002/jbm.a.37792. Epub 2024 Sep 5. J Biomed Mater Res A. 2025. PMID: 39237474
References
-
- Ingle J.N., Ahmann D.L., Green S.J., Edmonson J.H., Bisel H.F., Kvols L.K., Nichols W.C., Creagan E.T., Hahn R.G., Rubin J., et al. Randomized Clinical Trial of Diethylstilbestrol versus Tamoxifen in Postmenopausal Women with Advanced Breast Cancer. N. Engl. J. Med. 1981;304:16–21. doi: 10.1056/NEJM198101013040104. - DOI - PubMed
-
- Davies C., Godwin J., Gray R., Clarke M., Darby S., McGale P., Wang Y.C., Peto R., Pan H.C., Cutter D., et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

