Dysfunctional Electron Transport Chain Assembly in COXPD8
- PMID: 40863384
- PMCID: PMC12386341
- DOI: 10.3390/jcdd12080318
Dysfunctional Electron Transport Chain Assembly in COXPD8
Abstract
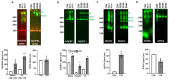
Combined oxidative phosphorylation deficiency type 8 (COXPD8) is an autosomal recessive mitochondrial disorder caused by a mutation of the nuclear encoded mitochondrial alanyl-tRNA synthetase gene (AARS2). Clinical manifestations of COXPD8 include lethal infantile hypertrophic cardiomyopathy, pulmonary hypoplasia, generalized muscle weakness, and neurological involvement. We report a patient with COXPD8 caused by two mutations in the AARS2 gene. The c.1738 C>G mutation has not been previously reported, while the c.2872 C>T mutation has been associated with pulmonary hypoplasia and hypertrophic cardiomyopathy. Cardiac tissue, obtained through the LungMAP program, showed that, compared to other patients of similar ages, these two mutations affect not only the assembly of functional monomeric complexes (Cx) I and IV of the electron transport chain (ETC) but also limit the formation of respiratory supercomplexes. This patient had altered expression of some ETC proteins but normal expression of several enzymes of the tricarboxylic acid cycle. We also show that one of the control/comparison patients had an undiagnosed ETC Cx IV deficiency. In conclusion, our data demonstrate that the two mutations of the AARS2 gene are associated with failed assembly of Cx I and Cx IV and reduced formation of respiratory supercomplexes of the ETC, likely leading to acute bioenergetic stress.
Keywords: COXPD8; electron transport chain; hypertrophic cardiomyopathy; mitochondrial disease; mitochondrial supercomplexes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Götz A., Tyynismaa H., Euro L., Ellonen P., Hyötyläinen T., Ojala T., Hämäläinen R.H., Tommiska J., Raivio T., Oresic M., et al. Exome sequencing identifies mitochondrial alanyl-tRNA synthetase mutations in infantile mitochondrial cardiomyopathy. Am. J. Hum. Genet. 2011;88:635–642. doi: 10.1016/j.ajhg.2011.04.006. - DOI - PMC - PubMed
-
- Sommerville E.W., Zhou X.-L., Oláhová M., Jenkins J., Euro L., Konovalova S., Hilander T., Pyle A., He L., Habeebu S., et al. Instability of the mitochondrial alanyl-tRNA synthetase underlies fatal infantile-onset cardiomyopathy. Hum. Mol. Genet. 2019;28:258–268. doi: 10.1093/hmg/ddy294. - DOI - PMC - PubMed
-
- Lapuente-Brun E., Moreno-Loshuertos R., Acín-Pérez R., Latorre-Pellicer A., Colás C., Balsa E., Perales-Clemente E., Quirós P.M., Calvo E., Rodríguez-Hernández M.A., et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science. 2013;340:1567–1570. doi: 10.1126/science.1230381. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

