From Screening to Therapy: A Personalized Approach to ROP in a National NICU Setting
- PMID: 40863450
- PMCID: PMC12387620
- DOI: 10.3390/jpm15080388
From Screening to Therapy: A Personalized Approach to ROP in a National NICU Setting
Abstract
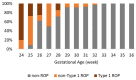
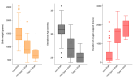
Aim: We aimed to investigate the incidence, treatment patterns, and associated risk factors of type 1 retinopathy of prematurity (ROP) in the only tertiary-level Neonatal Intensive Care Unit (NICU) in Cyprus. Methods: This retrospective study included all infants screened for ROP between January and December 2023. Data were collected from standardized NICU discharge summaries and included gestational age (GA), birth weight (BW), multiple birth, systemic infection, blood transfusion, oxygen therapy, surgical interventions, and ROP outcomes. Infants were categorized into non-ROP, non-type 1 ROP, and type 1 ROP groups. Statistical analysis was performed to identify differences in risk factor distribution. Results: Among 183 infants, 33 (18.0%) developed ROP, with 11 (6.0%) requiring treatment for type 1 ROP. All infants with type 1 ROP were born at ≤28 weeks GA and weighed <1501 g. Type 1 ROP was significantly associated with lower GA, lower BW, systemic infection, surgery, and prolonged oxygen support (p < 0.05). Six infants were treated with laser and three with intravitreal bevacizumab. No recurrence was observed in the anti-VEGF group during 18 months of follow-up. Two infants with aggressive ROP died before treatment. Conclusions: Type 1 ROP in Cyprus occurred exclusively in extremely preterm infants, associated with the cumulative effect of multiple risk factors. Laser remained the primary treatment, while anti-VEGF was used selectively with favorable outcomes. This study emphasizes the importance of tailoring ROP screening and treatment strategies based on individual neonatal risk profiles, supporting a personalized approach to neonatal ophthalmic care.
Keywords: Cyprus; ROP screening; anti-VEGF; laser photocoagulation; neonatal risk factors; prematurity; type 1 ROP.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Tsiropoulos G.N., Seliniotaki A.K., Haidich A.B., Ziakas N., Mataftsi A. Comparison of adverse events between intravitreal anti-VEGF and laser photocoagulation for treatment-requiring retinopathy of prematurity: A systematic review. Int. Ophthalmol. 2022;43:1027–1062. doi: 10.1007/s10792-022-02480-6. - DOI - PMC - PubMed
-
- Taher N.O., Ghaddaf A.A., Al-Ghamdi S.A., Homsi J.J., Al-Harbi B.J., Alomari L.K., Almarzouki H.S. Intravitreal Anti-vascular Endothelial Growth Factor Injection for Retinopathy of Prematurity: A Systematic Review and Meta-Analysis. Front. Med. 2022;9:884608. doi: 10.3389/fmed.2022.884608. - DOI - PMC - PubMed
-
- Wiecek E., Akula J.D., Vanderveen D.K., Mantagos I.S., Wu C., Curran A.L., De Bruyn H., Peterson B., Fulton A.B. Longitudinal Change of Refractive Error in Retinopathy of Prematurity Treated with Intravitreal Bevacizumab or Laser Photocoagulation. Am. J. Ophthalmol. 2022;240:252–259. doi: 10.1016/j.ajo.2022.03.020. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources