Phosphate Transport Through Homogeneous and Heterogeneous Anion-Exchange Membranes: A Chronopotentiometric Study for Electrodialytic Applications
- PMID: 40863591
- PMCID: PMC12388024
- DOI: 10.3390/membranes15080230
Phosphate Transport Through Homogeneous and Heterogeneous Anion-Exchange Membranes: A Chronopotentiometric Study for Electrodialytic Applications
Abstract
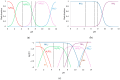
This study investigates the behavior of phosphate ion transport through two structurally distinct anion-exchange membranes-AMV (homogeneous) and HC-A (heterogeneous)-in an electrodialysis system under both static and stirred conditions at varying pH levels. Chronopotentiometric and current-voltage analyses were used to investigate the influence of pH and hydrodynamics on ion transport. Under underlimiting (ohmic) conditions, the AMV membrane exhibited simultaneous transport of H2PO4- and HPO42- ions at neutral and mildly alkaline pH, while such behavior was not verified at acidic pH and in all cases for the HC-A membrane. Under overlimiting current conditions, AMV favored electroconvection at low pH and exhibited significant water dissociation at high pH, leading to local pH shifts and chemical equilibrium displacement at the membrane-solution interface. In contrast, the HC-A membrane operated predominantly under strong electroconvective regimes, regardless of the pH value, without evidence of water dissociation or equilibrium change phenomena. Stirring significantly impacted the electrochemical responses: it altered the chronopotentiogram profiles through the emergence of intense oscillations in membrane potential drop at overlimiting currents and modified the current-voltage behavior by increasing the limiting current density, reducing electrical resistance, and compressing the plateau region that separates ohmic and overlimiting regimes. Additionally, both membranes showed signs of NH3 formation at the anodic-side interface under pH 7-8, associated with increased electrical resistance. These findings reveal distinct ionic transport characteristics and hydrodynamic sensitivities of the membranes, thus providing valuable insights for optimizing phosphate recovery via electrodialysis.
Keywords: electroconvection; electrodialysis; membrane potential drop; nutrient recovery; phosphorus; water dissociation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- Brownlie W.J., Sutton M.A., Cordell D., Reay D.S., Heal K.V., Withers P.J.A., Vanderbeck I., Spears B.M. Phosphorus price spikes: A wake-up call for phosphorus resilience. Front. Sustain. Food Syst. 2023;7:1088776. doi: 10.3389/fsufs.2023.1088776. - DOI
-
- European Commission Critical Raw Materials. 2023. [(accessed on 7 June 2025)]. Available online: https://single-market-economy.ec.europa.eu/sectors/raw-materials/areas-s....
-
- Akinnawo S.O. Eutrophication: Causes, consequences, physical, chemical and biological techniques for mitigation strategies. Environ. Chall. 2023;12:100733. doi: 10.1016/j.envc.2023.100733. - DOI
-
- Martín M., Hernández-Crespo C., Andrés-Doménech I., Benedito-Durá V. Fifty years of eutrophication in the Albufera lake (Valencia, Spain): Causes, evolution and remediation strategies. Ecol. Eng. 2020;155:105932. doi: 10.1016/j.ecoleng.2020.105932. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

