Dual Effects of Maternal Diet and Perinatal Organophosphate Flame Retardant Treatment on Offspring Development, Behavior and Metabolism
- PMID: 40863915
- PMCID: PMC12390071
- DOI: 10.3390/toxics13080639
Dual Effects of Maternal Diet and Perinatal Organophosphate Flame Retardant Treatment on Offspring Development, Behavior and Metabolism
Abstract
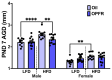
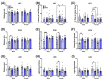
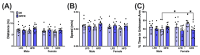
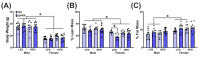
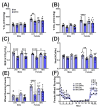
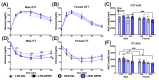
The maternal-fetal environment is influenced by multiple factors, including nutrition and environmental contaminants, which can impact long-term development. Perinatal exposure to organophosphate flame retardants (OPFRs) disrupts energy homeostasis and causes maladaptive behaviors in mice. Maternal obesity affects development by impairing blood-brain barrier (BBB) formation, influencing brain regions involved in energy regulation and behavior. This study examined the combined effects of maternal obesity and perinatal OPFR treatment on offspring development. Female mice were fed either a low-fat (LFD) or a high-fat diet (HFD) for 8 weeks, mated, and treated with either sesame oil or an OPFR mixture (tris(1,3-dichloro-2-propyl)phosphate, tricresyl phosphate, and triphenyl phosphate, 1 mg/kg each) from gestational day 7 to postnatal day 14. Results showed that both maternal diet and OPFR treatment disrupted blood-brain barrier integrity, energy balance, and reproductive gene expression in the hypothalamus of neonates. The expression of hepatic genes related to lipid and xenobiotic metabolism was also altered. In adulthood, LFD OPFR-treated female offspring exhibited increased avoidance behavior, while HFD OPFR-treated females demonstrated memory impairments. Metabolic assessments revealed decreased energy expenditure and nighttime activity in LFD OPFR-treated females. These findings suggest that maternal diet and OPFR treatment alter hypothalamic and liver gene expression in neonates, potentially leading to long-term metabolic and behavioral changes.
Keywords: anxiety; endocrine disruption; memory; neurodevelopment; organophosphate flame retardants.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
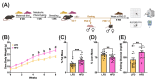
References
Grants and funding
LinkOut - more resources
Full Text Sources

