Functional and Proteomic Characterization of Acanthophis antarcticus Venom: Evidence of Fibrinogenolytic and Serine Peptidase Inhibitory Activities
- PMID: 40864081
- PMCID: PMC12389826
- DOI: 10.3390/toxins17080405
Functional and Proteomic Characterization of Acanthophis antarcticus Venom: Evidence of Fibrinogenolytic and Serine Peptidase Inhibitory Activities
Abstract
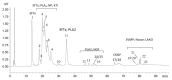
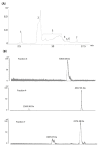
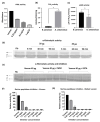
Acanthophis antarcticus, commonly known as the death adder, is a venomous Australian snake and a member of the Elapidae family. Due to its robust body and triangular head, it was historically misclassified as a viper. Its venom is known for neurotoxic, hemorrhagic, and hemolytic effects but displays low anticoagulant activity. Although key toxins such as three-finger toxins (3FTxs) and phospholipase A2 (PLA2) have been previously described, no study has integrated proteomic and functional analyses to date. In this study, we conducted a comprehensive characterization of A. antarcticus venom. Reverse-phase high-performance liquid chromatography (RP-HPLC) followed by LC-MS/MS enabled the identification of nine toxin families, with 3FTxs and PLA2 as the most abundant. Less abundant but functionally relevant toxins included Kunitz-type inhibitors, CRISP, SVMP, LAAO, NGF, natriuretic peptides, and nucleotidases, the latter being reported here for the first time based on proteomic evidence. Hydrophilic interaction chromatography (HILIC) coupled with MALDI-TOF was used to analyze polar, non-retained venom components, revealing the presence of low-molecular-weight peptides (2-4 kDa). Functional assays confirmed the enzymatic activity of HYAL, PLA2, and LAAO and, for the first time, demonstrated inhibitory activity on serine peptidases and fibrinogenolytic activity in the venom of this species. These findings expand our understanding of the biochemical and functional diversity of this venom.
Keywords: Acanthophis; Elapidae; fibrinogenolytic activity; metallopeptidases; proteomic; serine peptidase inhibitors; serine peptidases.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- M’Coy F. XVII.—Note on the Ancient and Recent Natural History of Victoria. Ann. Mag. Nat. Hist. 1862;9:137–150. doi: 10.1080/00222936208681197. - DOI
-
- Halford G.B. Thoughts, Observations and Experiments on the Action of Snakes Venom on the Blood: With an Appendix. Stillwell; Melbourne, Australia: 1894.
-
- Fairley N.H. The dentition and biting mechanism of Australian snakes. Med. J. Aust. 1929;1:313–327. doi: 10.5694/j.1326-5377.1929.tb14095.x. - DOI
-
- Macleay W.J. Papers and Proceedings of the Royal Society of Tasmania. University of Tasmania; Tasmania, Australia: 1885. Zoology of Australia; pp. 285–308. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

