Low Measles Seropositivity in Vaccinated Children
- PMID: 40864465
- PMCID: PMC12391999
- DOI: 10.1001/jamanetworkopen.2025.29409
Low Measles Seropositivity in Vaccinated Children
Abstract
Importance: India's goal of measles elimination remains unmet, as evidenced by significant recent outbreaks.
Objective: To identify seroprevalence rates among vaccinated children and to examine demographic factors that influence antibody responses.
Design, setting, and participants: This cross-sectional study included a highly vaccinated cohort of Indian children from the states of Kerala and Tamil Nadu between 2018 and 2023. Eligible children had received at least 2 doses of measles-containing vaccines; their mothers were recruited from various community settings, including local nursery and secondary schools, residential associations, and hospitals.
Exposure: Measles-specific antibodies were quantified in serum samples.
Main outcomes and measures: Measles-specific immunoglobulin G (IgG) and neutralizing antibodies were quantified in both children and mothers. Measles-specific IgM was also measured in children.
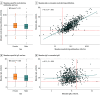
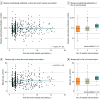
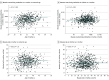
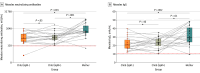
Results: The total cohort comprised 684 children (median age, 9 years [range, 4-18 years]; 348 male [50.1%]) and 544 mothers. All children received at least 2 doses of measles-containing vaccines; 435 children (63.6%) received a third dose, and 7 (1.0%) received a fourth dose. Among the children, 621 (90.8%) had positive measles-specific IgG, and 623 (91.5%) had protective neutralizing antibodies titers, with a strong correlation between measles-specific IgG and neutralizing antibodies (r = 0.73; P < .001). Female children exhibited significantly higher titers of both measles-specific IgG and neutralizing antibodies compared with male children. While IgG and neutralizing antibody titers remained stable over time and were not associated with the number of vaccine doses in children, neutralizing antibody titers increased with age in mothers, likely due to repeated viral exposure. Notably, in 20 families with at least 2 children, differential measles-specific IgM profiles were observed between siblings despite high IgG and neutralizing antibody titers, suggesting ongoing breakthrough infections.
Conclusions and relevance: In this cross-sectional study, substantial measles immunity gaps were found despite high vaccine coverage with evidence of breakthrough infections, posing significant challenges to India's measles elimination efforts. These results underscore the urgent need to strengthen India's immunization program and investigate the mechanisms driving suboptimal responses to measles vaccination; without addressing these immune response deficiencies, achieving measles elimination through increased vaccine coverage alone may remain unattainable in India.
Conflict of interest statement
Figures
References
-
- US Centers for Disease Control and Prevention . Global measles outbreaks. Updated July 9, 2025. Accessed March 26, 2025. https://www.cdc.gov/global-measles-vaccination/data-research/global-meas....
-
- Murhekar MV, Gupta N, Hasan AZ, et al. Evaluating the effect of measles and rubella mass vaccination campaigns on seroprevalence in India: a before-and-after cross-sectional household serosurvey in four districts, 2018-2020. Lancet Glob Health. 2022;10(11):e1655-e1664. doi: 10.1016/S2214-109X(22)00379-5 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

