Mitochondrial ROS drive foam cell formation via STAT5 signaling in atherosclerosis
- PMID: 40864703
- PMCID: PMC12383252
- DOI: 10.1126/sciadv.adw9952
Mitochondrial ROS drive foam cell formation via STAT5 signaling in atherosclerosis
Abstract
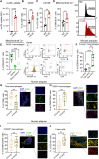
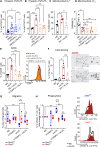
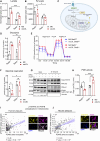
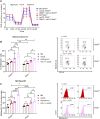
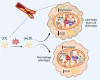
Macrophage-to-foam cell transition is an integral part of atherosclerotic plaque progression. Particularly, oxidized low-density lipoprotein (oxLDL) is a driving factor in foam cell formation, altering macrophage function and metabolism. The aim of our research was to understand the impact of oxLDL-induced mitochondrial reactive oxygen species on macrophage-to-foam cell differentiation. We demonstrate that macrophage oxLDL-derived superoxide modulates mitochondrial metabolic reprogramming, facilitating foam cell formation. Mechanistically, mitochondrial superoxide drives signal transducers and activators of transcription 5 (STAT5) activation, leading to reduced tricarboxylic acid cycle activity. In parallel, mitochondrial superoxide enhances chromatin accessibility at STAT5 target genes, establishing a distinct STAT5 signaling signature in foam cells ex vivo and in human and mouse plaques in vivo. Inhibition of STAT5 during atherosclerosis progression prevents the differentiation of macrophages to mature Trem2hiGpnmbhi foam cells. Collectively, our data describe an oxLDL-induced, mitochondrial superoxide-dependent STAT5 activation that leads to a self-amplifying feedback loop of reciprocal mitochondrial superoxide production and STAT5 activation, ultimately driving macrophage-to-foam cell transition.
Figures



References
-
- Patial S., Sharma A., Raj K., Shukla G., Atherosclerosis: Progression, risk factors, diagnosis, treatment, probiotics and synbiotics as a new prophylactic hope. Microbe 5, 100212 (2024).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

