ACTN3 genotype influences androgen response in developing murine skeletal muscle
- PMID: 40864710
- PMCID: PMC12383265
- DOI: 10.1126/sciadv.adw1059
ACTN3 genotype influences androgen response in developing murine skeletal muscle
Abstract
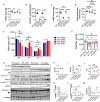
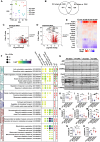
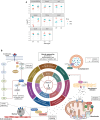
Androgens act through androgen receptor (AR) to maintain muscle mass. Evidence suggests that this pathway is influenced by "the gene for speed," ACTN3 (α-actinin-3). Given that one in five people lack α-actinin-3, it is possible that they may respond to androgens differently. Here, we show that α-actinin-3 deficiency decreases AR in muscles of mice and humans (in males and females) and that AR positively correlates with α-actinin-3 expression in a dosage-dependent manner. α-Actinin-3 deficiency exacerbates gastrocnemius mass loss with androgen deprivation in male mice and stunts the muscle growth response to dihydrotestosterone in female mice at the onset of puberty. This is mediated by differential activation of pathways regulating amino acid metabolism, intracellular transport, autophagy, mitochondrial activity, MAPK, and calcineurin signaling, likely driven by seven key genes that are both androgen sensitive and α-actinin-3-dependent in expression. Our results highlight a role for ACTN3 as a regulator of muscle mass and a genetic modifier of androgen action in skeletal muscle.
Figures




References
-
- Handelsman D. J., Androgen misuse and abuse. Endocr. Rev. 42, 457–501 (2021). - PubMed
-
- Huang G., Bhasin S., Pencina K., Cheng M., Jasuja R., Circulating dihydrotestosterone, testosterone, and free testosterone levels and dihydrotestosterone-to-testosterone ratios in healthy women across the menstrual cycle. Fertil. Steril. 118, 1150–1158 (2022). - PubMed
-
- de Rooy C., Grossmann M., Zajac J. D., Cheung A. S., Targeting muscle signaling pathways to minimize adverse effects of androgen deprivation. Endocr. Relat. Cancer 23, R15–R26 (2016). - PubMed
-
- Qin W., Pan J., Wu Y., Bauman W. A., Cardozo C., Anabolic steroids activate calcineurin-NFAT signaling and thereby increase myotube size and reduce denervation atrophy. Mol. Cell. Endocrinol. 399, 336–345 (2015). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

