CD99-mediated immunological synapse formation potentiates CAR-T cell function
- PMID: 40866332
- PMCID: PMC12391334
- DOI: 10.1038/s41467-025-63184-w
CD99-mediated immunological synapse formation potentiates CAR-T cell function
Abstract
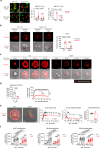
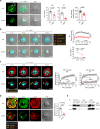
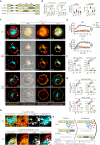
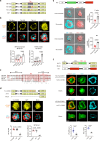
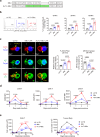
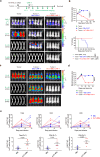
Despite the efficacy of chimeric antigen receptor (CAR)-T cells in selected hematological malignancies, further improvement on CAR-T designs is still desirable. We hypothesize that modifying the CAR structure to enhance immunological synapse (IS) stabilization and CAR target-binding may be a feasible strategy. Here we show that the membrane protein, CD99, is critical for IS formation in T cells by mediating actin-microtubule interaction. CD99 deficiency abolishes IS formation and prevents effective in vivo T cell immunity. Mechanistically, CD99 interacts with microtubules and actins through the transmembrane and cytoplasmic domains, respectively, with which myosin and IQGAP1 interact. As such, incorporating the transmembrane and juxtamembrane domains of CD99 into the CAR structure enhances IS formation and improves the therapeutic efficacy of human CAR-T cells against lymphoma in immune-deficient mice. Our data thus suggest that CD99-mediated IS stabilization may help improve CAR design and efficacy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: G.N., H.R.Y., H.B.P., J.E.L., K.C., and E.Y.C. are inventors on a filed patent related to the CAR-T cells. K.C. and E.Y.C. are scientific co-founders and shareholders of Ticaros. H.R.Y., H.B.P., and J.E.L. are employees of Ticaros currently. The remaining authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
- RS-2024-00405619/National Research Foundation of Korea (NRF)
- 2021R1A4A1029097/National Research Foundation of Korea (NRF)
- 2024RS-2023-00260454/National Research Foundation of Korea (NRF)
- RS-2024-00400897/National Research Foundation of Korea (NRF)
- 2021R1A4A1029097/National Research Foundation of Korea (NRF)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

