Remodeling adipocytes' lipid metabolism with a polycation loaded enzyme-active framework reverses osteoporotic bone marrow
- PMID: 40866384
- PMCID: PMC12391307
- DOI: 10.1038/s41467-025-63376-4
Remodeling adipocytes' lipid metabolism with a polycation loaded enzyme-active framework reverses osteoporotic bone marrow
Abstract
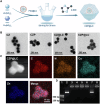
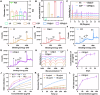
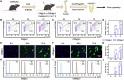
The function of osteoporosis-induced bone marrow adipocyte (BMAds) accumulation remains inadequately understood. Here, we analyze bone marrow lipidomic data and reveal that BMAds deteriorate the skeletal microenvironment by secreting large amounts of lipids, altering the senescence status of neighboring cells by affecting their mitochondrial function. To specifically target BMAds under osteoporotic conditions, we design a polycation-loaded biomimetic dual-site framework (CZP@LC) that interferes with lipid crosstalk between BMAds and neighboring bone marrow cells. Shutting down abnormal lipid metabolism and secretion in adipocytes mitigates mitochondrial dysfunction in neighboring cells, which prevents bone marrow cells from senescing. The inhibition of lipid synthesis in BMAds blocks bone marrow stromal cells from differentiating into adipocytes, interrupting the vicious cycle. Moreover, interruption of lipid communication rescues osteoblasts from mitochondrial dysfunction-induced senescence and restores osteogenesis. Here we demonstrate the metabolic mechanisms of BMAds and lipid crosstalk in osteoporosis, provide a potential avenue for targeted biotherapy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures







References
-
- Wang, Y. et al. Bone marrow adipocyte: origin, biology and relationship with hematological malignancy. Int. J. Lab. Hematol.46, 10–19 (2024). - PubMed
-
- Sulston, R. J. & Cawthorn, W. P. Bone marrow adipose tissue as an endocrine organ: close to the bone? Horm. Mol. Biol. Clin. Investig.28, 21–38 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

