Stepwise approach to alzheimer's disease diagnosis in primary care using cognitive screening, risk factors, neuroimaging and plasma biomarkers
- PMID: 40866545
- PMCID: PMC12391538
- DOI: 10.1038/s41598-025-17394-3
Stepwise approach to alzheimer's disease diagnosis in primary care using cognitive screening, risk factors, neuroimaging and plasma biomarkers
Abstract
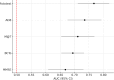
Early identification of Alzheimer's disease (AD) pathology is essential for timely intervention, particularly in primary care. We evaluated the diagnostic performance of a scalable, multimodal framework in a real-world, population-based cohort. A total of 277 community-dwelling individuals aged ≥ 60 years from the STOP-ALZHEIMER DEBA study (Basque Country, Spain) underwent brief cognitive screening (MMSE, M@T, Fototest, AD8) with optimized cut-offs, along with clinical risk assessment. Among them, 181 participants also completed structural MRI, plasma biomarker profiling (p-tau181, Aβ42/40, GFAP, NfL), and cerebrospinal fluid (CSF) analysis. We assessed performance for detecting cognitive impairment, CSF amyloid positivity (A+), and combined amyloid-tau positivity (A + T+). Optimized cognitive tests showed moderate accuracy (AUC 0.66-0.77), with the Fototest performing best. For biological outcomes, GFAP and p-tau181 had the highest predictive value (AUCs: 0.813 and 0.755 for A+; 0.852 and 0.710 for A + T+), and their combination further improved accuracy (AUC = 0.842). Fully adjusted models incorporating optimized cognitive scores, plasma biomarkers, APOE genotype, MRI, and demographics achieved high diagnostic performance (AUC = 0.886 for A+; 0.893 for A + T+). Results were consistent across sex and age strata. These findings support a stepwise diagnostic strategy combining brief, minimally invasive tools to enhance early AD detection in community settings.
Keywords: ATN framework; Alzheimer’s disease; Cognitive screening; Mild cognitive impairment; Plasma biomarkers; Primary care.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
- PI21/00718/Instituto de Salud Carlos III
- PI21/00718/Instituto de Salud Carlos III
- PI21/00718/Instituto de Salud Carlos III
- PI21/00718/Instituto de Salud Carlos III
- PI21/00718/Instituto de Salud Carlos III
- PI21/00718/Instituto de Salud Carlos III
- PI21/00718/Instituto de Salud Carlos III
- PI21/00718/Instituto de Salud Carlos III
- PI21/00718/Instituto de Salud Carlos III
- PI21/00718/Instituto de Salud Carlos III
- PI21/00718/Instituto de Salud Carlos III
- BIO22/ALZ/014/Berrikuntza + Ikerketa + Osasuna Eusko Fundazioa
- BIO22/ALZ/014/Berrikuntza + Ikerketa + Osasuna Eusko Fundazioa
- BIO22/ALZ/014/Berrikuntza + Ikerketa + Osasuna Eusko Fundazioa
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

