One-shot design of functional protein binders with BindCraft
- PMID: 40866699
- PMCID: PMC12507698
- DOI: 10.1038/s41586-025-09429-6
One-shot design of functional protein binders with BindCraft
Abstract
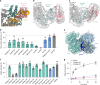
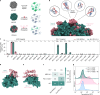
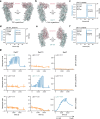
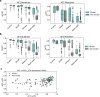
Protein-protein interactions are at the core of all key biological processes. However, the complexity of the structural features that determine protein-protein interactions makes their design challenging. Here we present BindCraft, an open-source and automated pipeline for de novo protein binder design with experimental success rates of 10-100%. BindCraft leverages the weights of AlphaFold2 (ref. 1) to generate binders with nanomolar affinity without the need for high-throughput screening or experimental optimization, even in the absence of known binding sites. We successfully designed binders against a diverse set of challenging targets, including cell-surface receptors, common allergens, de novo designed proteins and multi-domain nucleases, such as CRISPR-Cas9. We showcase the functional and therapeutic potential of designed binders by reducing IgE binding to birch allergen in patient-derived samples, modulating Cas9 gene editing activity and reducing the cytotoxicity of a foodborne bacterial enterotoxin. Last, we use cell-surface-receptor-specific binders to redirect adeno-associated virus capsids for targeted gene delivery. This work represents a significant advancement towards a 'one design-one binder' approach in computational design, with immense potential in therapeutics, diagnostics and biotechnology.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: K.H.G., L.V., B.J.Y. and A.M.W. are employees of Visterra Inc., USA. The remaining authors declare no competing interests.
Figures








Update of
-
BindCraft: one-shot design of functional protein binders.bioRxiv [Preprint]. 2025 Apr 25:2024.09.30.615802. doi: 10.1101/2024.09.30.615802. bioRxiv. 2025. Update in: Nature. 2025 Oct;646(8084):483-492. doi: 10.1038/s41586-025-09429-6. PMID: 39677777 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

