A compressed hierarchy for visual form processing in the tree shrew
- PMID: 40866712
- PMCID: PMC12545169
- DOI: 10.1038/s41586-025-09441-w
A compressed hierarchy for visual form processing in the tree shrew
Abstract
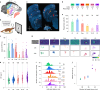
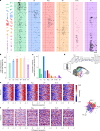
Our knowledge of the brain processes that govern vision is largely derived from studying primates, whose hierarchically organized visual system1 inspired the architecture of deep neural networks2. This raises questions about the universality of such hierarchical structures. Here we examined the large-scale functional organization for vision in one of the closest living relatives to primates, the tree shrew. We performed Neuropixels recordings3,4 across many cortical and thalamic areas spanning the tree shrew ventral visual system while presenting a large battery of visual stimuli in awake tree shrews. We found that receptive field size, response latency and selectivity for naturalistic textures, compared with spectrally matched noise5, all increased moving anteriorly along the tree shrew visual pathway, consistent with a primate-like hierarchical organization6,7. However, tree shrew area V2 already harboured a high-level representation of complex objects. First, V2 encoded a complete representation of a high-level object space8. Second, V2 activity supported the most accurate object decoding and reconstruction among all tree shrew visual areas. In fact, object decoding accuracy from tree shrew V2 was comparable to that in macaque posterior IT and substantially higher than that in macaque V2. Finally, starting in V2, we found strongly face-selective cells resembling those reported in macaque inferotemporal cortex9. Overall, these findings show how core computational principles of visual form processing found in primates are conserved, yet hierarchically compressed, in a small but highly visual mammal.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures









References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

