Cocaine chemogenetics blunts drug-seeking by synthetic physiology
- PMID: 40866713
- PMCID: PMC12527922
- DOI: 10.1038/s41586-025-09427-8
Cocaine chemogenetics blunts drug-seeking by synthetic physiology
Abstract
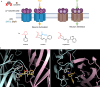
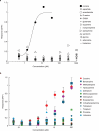
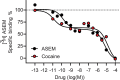
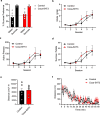
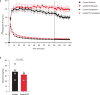
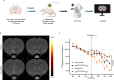
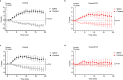
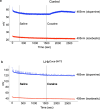
Chemical feedback is ubiquitous in physiology but is challenging to study without perturbing basal functions. One example is addictive drugs, which elicit a positive-feedback cycle of drug-seeking and ingestion by acting on the brain to increase dopamine signalling1-3. However, interfering with this process by altering basal dopamine also adversely affects learning, movement, attention and wakefulness4. Here, inspired by physiological control systems, we developed a highly selective synthetic physiology approach to interfere with the positive-feedback cycle of addiction by installing a cocaine-dependent opposing signalling process into this body-brain signalling loop. We used protein engineering to create cocaine-gated ion channels that are selective for cocaine over other drugs and endogenous molecules. Expression of an excitatory cocaine-gated channel in the rat lateral habenula, a brain region that is normally inhibited by cocaine, suppressed cocaine self-administration without affecting food motivation. This artificial cocaine-activated chemogenetic process reduced the cocaine-induced extracellular dopamine rise in the nucleus accumbens. Our results show that cocaine chemogenetics is a selective approach for countering drug reinforcement by clamping dopamine release in the presence of cocaine. In the future, chemogenetic receptors could be developed for additional addictive drugs or hormones and metabolites, which would facilitate efforts to probe their neural circuit mechanisms using a synthetic physiology approach. As these chemogenetic ion channels are specific for cocaine over natural rewards, they may also offer a route towards gene therapies for cocaine addiction.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.M.S. and C.J.M. have a pending patent application on this technology (US patent application 20240002463-A1) as well as additional issued patents on related chemogenetic technologies. S.M.S. is a consultant for Kriya Therapeutics, which is focused on therapeutic applications of chemogenetics. M.M. is a principal investigator on a cooperative research and development agreement between NIDA and Kriya Therapeutics. The other authors declare no competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

