β-elemene promotes ferroptosis to improve the sensitivity of imatinib in gastrointestinal stromal tumours by targeting N6AMT1
- PMID: 40866937
- PMCID: PMC12390768
- DOI: 10.1002/ctm2.70438
β-elemene promotes ferroptosis to improve the sensitivity of imatinib in gastrointestinal stromal tumours by targeting N6AMT1
Abstract
Background: Imatinib has been widely used in gastrointestinal stromal tumours and significantly improved the prognosis of GIST patients, but approximately half of patients develop acquired treatment resistance, highlighting the urgency for novel therapeutic strategies.
Methods: A variety of bioinformatic tools and laboratory experiments, RNA sequencing, animal models and the thermal proteome profiling assay were employed to validate our findings and investigate the antitumour effects of β-elemene.
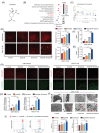
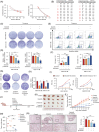
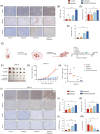
Results: We found that imatinib-resistant GIST was associated with negative regulation of ferroptosis activity, and inducing ferroptosis can enhance the sensitivity of resistant cells to imatinib. Furthermore, we found that β-elemene enhances imatinib sensitivity in imatinib-resistant GIST cells through inducing ferroptosis. Moreover, the combination treatment of β-elemene and imatinib showed significantly increased antitumour efficacy, compared to each monotherapy, both in vitro and in vivo. Mechanistically, β-elemene specifically targets N6AMT1, inhibiting its transcriptional repression function and activating the nuclear factor erythroid 2-related factor 2 (NRF2)-HMOX1 signalling pathway to induce ferroptosis.
Conclusion: Β-elemene can target N6AMT1 and promote ferroptosis by increasing the expression of NRF2 and HMOX1. These findings suggest β-elemene as a prospective therapeutic strategy to improve the sensitivity of imatinib in gastrointestinal stromal tumours.
Key points: l Imatinib resistance is associated with ferroptosis activity in GIST. l Combination of β-elemene and imatinib effectively treats gastrointestinal stromal tumours both in vivo and in vitro. l β-elemene promotes imatinib sensitivity in GIST through ferroptosis. l N6AMT1 is a potential target of β-elemene. l β-elemene targets N6AMT1 to promote imatinib sensitivity in imatinib-resistant GIST cells via the NRF2/HMOX1 axis.
Keywords: ROS; ferroptosis; gastrointestinal stromal tumours; imatinib resistance; β‐elemene.
© 2025 The Author(s). Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
AT101 Suppresses Gastrointestinal Stromal Tumor Growth and Promotes Apoptosis via YAP/TAZ-CCND1 and FBXW7-MCL1 Axes.Ann Surg Oncol. 2025 Aug;32(8):5991-6004. doi: 10.1245/s10434-025-17247-3. Epub 2025 Mar 27. Ann Surg Oncol. 2025. PMID: 40148719
-
KITlow Cells Mediate Imatinib Resistance in Gastrointestinal Stromal Tumor.Mol Cancer Ther. 2021 Oct;20(10):2035-2048. doi: 10.1158/1535-7163.MCT-20-0973. Epub 2021 Aug 10. Mol Cancer Ther. 2021. PMID: 34376580 Free PMC article.
-
Interpretable artificial intelligence to optimise use of imatinib after resection in patients with localised gastrointestinal stromal tumours: an observational cohort study.Lancet Oncol. 2024 Aug;25(8):1025-1037. doi: 10.1016/S1470-2045(24)00259-6. Epub 2024 Jul 5. Lancet Oncol. 2024. PMID: 38976997 Free PMC article.
-
Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation.Health Technol Assess. 2005 Jul;9(25):1-142. doi: 10.3310/hta9250. Health Technol Assess. 2005. PMID: 15985189
-
Getting the GIST of long non-coding RNA.Biochim Biophys Acta Rev Cancer. 2025 Sep;1880(4):189357. doi: 10.1016/j.bbcan.2025.189357. Epub 2025 May 28. Biochim Biophys Acta Rev Cancer. 2025. PMID: 40446968 Review.
References
MeSH terms
Substances
Grants and funding
- 2023A1515010417/Natural Science Foundation of Guangdong Province
- Y-Young2022-0023/Beijing Xisike Clinical Oncology Research Foundation
- Y-Gilead2024-PT-0006/Beijing Xisike Clinical Oncology Research Foundation
- Chih Kuang Scholarship for Outstanding Young Physician-Scientists of Sun Yat-sen University Cancer Center
LinkOut - more resources
Full Text Sources
