Dual functionality of MDM2 in PROTACs expands the horizons of targeted protein degradation
- PMID: 40866949
- PMCID: PMC12392632
- DOI: 10.1186/s40364-025-00826-7
Dual functionality of MDM2 in PROTACs expands the horizons of targeted protein degradation
Abstract
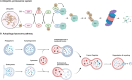
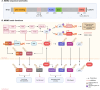
The evolution of targeted protein degradation (TPD) has been significantly propelled by the advent of proteolysis-targeting chimeras (PROTACs), which utilize heterobifunctional molecules to facilitate the ubiquitination-mediated degradation of previously "undruggable" proteins. Mouse double minute 2 (MDM2), which is often overexpressed in various diseases and plays a crucial role in regulating key pathways like p53, emerges as an exemplary candidate for therapeutic exploitation within the TPD realm, serving both as an intrinsic E3 ligase and as a direct protein of interest (POI). By harnessing MDM2's inherent E3 ligase activity, PROTACs have been designed to efficiently degrade specific POIs, achieving substantial success in both in vitro and in vivo studies. Alternatively, PROTACs have been developed to directly target MDM2 itself, offering new approaches for therapeutic intervention. Recent research has yielded valuable strategies for optimizing MDM2-harnessing and MDM2-targeted PROTAC designs, concentrating on warhead selection of POI, linker length and composition optimization, and the choice among various E3 ligases and their corresponding recruiters. These advancements not only broaden the scope of PROTAC technologies but also expedite the development of MDM2-based therapies, inspiring approaches for disease treatment.
Keywords: Bridged PROTAC; Homo-PROTAC; MDM2; PROTAC; TPD.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: The content of this manuscript has not been previously published and is not under consideration for publication elsewhere. All the authors agree to the content of the paper and their being listed as a co-author of the paper. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Epigenetic therapy meets targeted protein degradation: HDAC-PROTACs in cancer treatment.Future Med Chem. 2025 Jul;17(14):1725-1737. doi: 10.1080/17568919.2025.2533113. Epub 2025 Jul 16. Future Med Chem. 2025. PMID: 40667573 Review.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
PROTAC-Based Antivirals for Respiratory Viruses: A Novel Approach for Targeted Therapy and Vaccine Development.Microorganisms. 2025 Jul 2;13(7):1557. doi: 10.3390/microorganisms13071557. Microorganisms. 2025. PMID: 40732065 Free PMC article. Review.
-
Accurate PROTAC-targeted degradation prediction with DegradeMaster.Bioinformatics. 2025 Jul 1;41(Supplement_1):i342-i351. doi: 10.1093/bioinformatics/btaf191. Bioinformatics. 2025. PMID: 40662822 Free PMC article.
-
Synthesis and Application of BRD4-Targeting ByeTACs (Bypassing E-Ligase Targeting Chimeras).Curr Protoc. 2025 Aug;5(8):e70196. doi: 10.1002/cpz1.70196. Curr Protoc. 2025. PMID: 40788235
References
-
- Hipp MS, Kasturi P, Hartl FU. The proteostasis network and its decline in ageing. Nat Rev Mol Cell Biol. 2019;20:421–35. - PubMed
-
- Elsasser S, Elia LP, Morimoto RI, Powers ET, Finley D, Costa B, Budron M, Tokuno Z, Wang S, Iyer RG, et al. A comprehensive enumeration of the human proteostasis network. 2. Components of the autophagy-lysosome pathway. bioRxiv 2023;533675.
Publication types
Grants and funding
- 82472394 and 82172386 to C.L./National Natural Science Foundation Council of China
- 2022A1515012164 to C.L./Basic and Applied Basic Research Foundation of Guangdong Province
- JCYJ20210324104201005 and SGDX20240115112400001 to C.L./Shenzhen Science and Technology Program
- 2024YFC3506200 and 2024YFC3506205 to C.L./Key Technologies Research and Development Program
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

