Anticancer effects of folic acid-functionalized covalent organic framework containing doxorubicin on SW480 colon cancer cells: a promising tool for drug targeted delivery
- PMID: 40866979
- PMCID: PMC12382180
- DOI: 10.1186/s12896-025-01027-8
Anticancer effects of folic acid-functionalized covalent organic framework containing doxorubicin on SW480 colon cancer cells: a promising tool for drug targeted delivery
Abstract
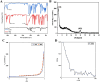
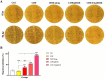
Colorectal cancer is one of the deadliest forms of gastrointestinal cancer, with conventional treatments often facing significant limitations. As a result, new approaches, particularly in targeted drug delivery, have shown great promise. In this study, the COF-FA@DOX nanocarrier was developed, where covalent organic frameworks (COFs) were functionalized with folic acid (FA) and then loaded with Doxorubicin (DOX). The as-synthesized COF-FA@DOX nanocarrier was characterized using different techniques. To assess its anticancer effectiveness, MTT, flow cytometry, and scratch assays were conducted on SW480 and HUVEC cells to examine cell viability, cellular uptake, cell cycle progression, apoptosis, and cell migration, respectively. The obtained results demonstrated that the COF-FA@DOX nanocarrier was efficiently internalized by cancer cells and showed significantly higher cytotoxicity compared to other synthesized nanocarrier groups and free DOX drug. Moreover, the COF-FA@DOX nanocarrier caused cell cycle arrest, induced apoptosis, and inhibited cell migration at lower doses than the free DOX drug. Altogether, these findings suggest that the COF-FA@DOX nanocarrier is an effective and promising drug delivery system for DOX in colorectal cancer, potentially enhancing the therapeutic efficacy of DOX drug while minimizing side effects through targeted delivery. Further investigation is required to assess their efficacy in vivo and discover potential clinical applications.
Keywords: Colorectal cancer; Covalent organic frameworks; Doxorubicin; Drug delivery; Folic acid; Targeted therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests. Declaration of competing interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures










Similar articles
-
Magnetic targeting enhances nanostructured covalent organic framework-doxorubicin cellular uptake in triple-negative breast cancer: A model for receptor-deficient cancers.Int J Biol Macromol. 2025 Sep;323(Pt 2):147169. doi: 10.1016/j.ijbiomac.2025.147169. Epub 2025 Aug 26. Int J Biol Macromol. 2025. PMID: 40876693
-
Zinc-cystine bio-MOF coated with folic acid-modified chitosan nanogel for targeted pH/glutathione dual-responsive drug delivery.Int J Biol Macromol. 2025 Sep;321(Pt 4):146459. doi: 10.1016/j.ijbiomac.2025.146459. Epub 2025 Jul 30. Int J Biol Macromol. 2025. PMID: 40749914
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.Health Technol Assess. 2006 Mar;10(9):1-132. iii-iv. doi: 10.3310/hta10090. Health Technol Assess. 2006. PMID: 16545208
References
-
- Kuipers EJ, Rösch T, Bretthauer M. Colorectal cancer screening–optimizing current strategies and new directions. Nat Rev Clin Oncol. 2013;10(3):130–42. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

