Redox-Regulated Pathways in Glioblastoma Stem-like Cells: Mechanistic Insights and Therapeutic Implications
- PMID: 40867215
- PMCID: PMC12384697
- DOI: 10.3390/brainsci15080884
Redox-Regulated Pathways in Glioblastoma Stem-like Cells: Mechanistic Insights and Therapeutic Implications
Abstract
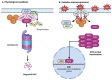
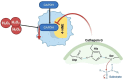
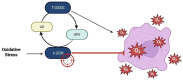
Glioblastoma (GBM) is the most aggressive primary brain tumor, characterized by rapid proliferation, invasiveness, therapeutic resistance, and an immunosuppressive tumor microenvironment. A subpopulation of glial stem-like cells (GSCs) within GBM tumors contributes significantly to tumor initiation, progression, and relapse, displaying remarkable adaptability to oxidative stress and metabolic reprogramming. Recent evidence implicates the atypical kinases RIOK1 and RIOK2 in promoting GBM growth and proliferation through their interaction with oncogenic pathways such as AKT and c-Myc. Concurrently, the redox-sensitive Nrf2/Keap1 axis regulates antioxidant defenses and supports GSC survival and chemoresistance. Additionally, aberrant activation of the canonical Wnt/β-catenin pathway in GSCs enhances their self-renewal, immune evasion, and resistance to standard therapies, particularly under oxidative stress conditions. This review integrates current knowledge on how redox homeostasis and key signaling pathways converge to sustain GSC maintenance and GBM malignancy. Finally, we discuss emerging redox-based therapeutic strategies designed to target GSC resilience, modulate the tumor immune microenvironment, and surmount treatment resistance.
Keywords: cancer stem cells; glial stem-like cells; glioblastoma; glioma; oxidative stress; redox-targeted therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Angiogenesis and Resistance Mechanisms in Glioblastoma: Targeting Alternative Vascularization Pathways to Overcome Therapy Resistance.Curr Pharm Des. 2025 Jul 22. doi: 10.2174/0113816128367551250703122830. Online ahead of print. Curr Pharm Des. 2025. PMID: 40698688
-
Glioma Stem Cells as Promoter of Glioma Progression: A Systematic Review of Molecular Pathways and Targeted Therapies.Int J Mol Sci. 2024 Jul 22;25(14):7979. doi: 10.3390/ijms25147979. Int J Mol Sci. 2024. PMID: 39063221 Free PMC article.
-
Autophagy-related CMTM6 promotes glioblastoma progression by activating Wnt/β-catenin pathway and acts as an onco-immunological biomarker.J Gene Med. 2024 May;26(5):e3685. doi: 10.1002/jgm.3685. J Gene Med. 2024. PMID: 38686653
-
SENP1-Mediated deSUMOylation Regulates the Tumor Remodeling of Glioma Stem Cells Under Hypoxic Stress.Technol Cancer Res Treat. 2024 Jan-Dec;23:15330338241257490. doi: 10.1177/15330338241257490. Technol Cancer Res Treat. 2024. PMID: 38803001 Free PMC article.
-
The Pivotal Role of NF-κB in Glioblastoma: Mechanisms of Activation and Therapeutic Implications.Int J Mol Sci. 2025 Aug 15;26(16):7883. doi: 10.3390/ijms26167883. Int J Mol Sci. 2025. PMID: 40869207 Free PMC article. Review.
References
-
- Aiman W., Gasalberti D.P., Rayi A. StatPearls. StatPearls Publishing; St. Petersburg, FL, USA: 2025. Low-Grade Gliomas. - PubMed
-
- Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncology. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

