Urinary microRNAs as Prognostic Biomarkers for Predicting the Efficacy of Immune Checkpoint Inhibitors in Patients with Urothelial Carcinoma
- PMID: 40867269
- PMCID: PMC12385101
- DOI: 10.3390/cancers17162640
Urinary microRNAs as Prognostic Biomarkers for Predicting the Efficacy of Immune Checkpoint Inhibitors in Patients with Urothelial Carcinoma
Abstract
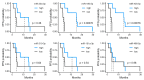
Background: Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of urothelial carcinoma (UC); however, their efficacy varies among patients. Identifying reliable biomarkers to predict response to ICIs remains challenging. We aimed to explore urinary microRNAs (miRNAs) as potential biomarkers for predicting ICI efficacy in patients with UC. Methods: We prospectively collected urinary samples from patients with UC before ICI initiation and investigated the predictive value of urinary miRNAs in patients with UC receiving ICIs. The expression levels of these miRNAs in pretreated urine samples were analyzed using next-generation sequencing. The patients were categorized as responders (those with stable disease or better for >6 months) or nonresponders (those who experienced disease progression within 6 months of treatment initiation). Urinary miRNA levels were compared between the groups to assess their potential as predictive biomarkers. Results: Elevated expression of miR-185-5p and miR-425-5p in the responder group was significantly associated with improved overall and progression-free survival in patients with bladder cancer treated with ICIs (p < 0.05). Conversely, higher levels of miR-30a-5p and miR-542-3p in the nonresponder group were correlated with a poorer response to ICIs, suggesting a potential role in immune resistance. Conclusions: miR-185-5p and miR-425-5p can serve as predictive biomarkers of favorable ICI efficacy in bladder cancer, whereas miR-30a-5p and miR-542-3p could be associated with resistance mechanisms. These findings highlight the potential of miRNA-based biomarkers, particularly those found in urine samples, to guide personalized immunotherapeutic strategies for UC treatment.
Keywords: biomarker; bladder cancer; immune check point inhibitors; miRNA; urothelial carcinoma.
Conflict of interest statement
M. Mizunuma and Y. Ichikawa are board members and shareholders of Craif Inc. A. Satomura, Y. Ando, and M. Mikami are employees of Craif Inc. and hold stock options in the company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Immune landscape and TAM density in endometrial cancer: implications for immune checkpoint inhibitors efficacy.Ther Adv Med Oncol. 2025 Aug 8;17:17588359251347364. doi: 10.1177/17588359251347364. eCollection 2025. Ther Adv Med Oncol. 2025. PMID: 40786594 Free PMC article.
-
Interplay between tumor mutation burden and the tumor microenvironment predicts the prognosis of pan-cancer anti-PD-1/PD-L1 therapy.Front Immunol. 2025 Jul 24;16:1557461. doi: 10.3389/fimmu.2025.1557461. eCollection 2025. Front Immunol. 2025. PMID: 40777041 Free PMC article.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
MicroRNA-126-3p as a predictive biomarker for patients with primary biliary cholangitis refractory to ursodeoxycholic acid.World J Gastroenterol. 2025 Aug 21;31(31):109828. doi: 10.3748/wjg.v31.i31.109828. World J Gastroenterol. 2025. PMID: 40901689 Free PMC article.
-
Immune checkpoint inhibitors plus platinum-based chemotherapy compared to platinum-based chemotherapy with or without bevacizumab for first-line treatment of older people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2024 Aug 13;8(8):CD015495. doi: 10.1002/14651858.CD015495. Cochrane Database Syst Rev. 2024. PMID: 39136258 Free PMC article.
References
-
- Rosenberg J.E., Hoffman-Censits J., Powles T., van der Heijden M.S., Balar A.V., Necchi A., Dawson N., O’Donnell P.H., Balmanoukian A., Loriot Y., et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

