Regulators of Cancer Progression: Succinylation
- PMID: 40867281
- PMCID: PMC12384786
- DOI: 10.3390/cancers17162652
Regulators of Cancer Progression: Succinylation
Abstract
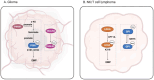
Lysine succinylation is a recently discovered post-translational protein modification, the process of which requires the participation of various enzymes. The close association between cancer and protein post-translational modifications (PTMs), such as acetylation and phosphorylation, has been extensively investigated and well-established. In recent years, growing attention has been directed toward the role of succinylation in cancer progression. Accumulating evidence demonstrates that protein succinylation and desuccinylation play critical roles in promoting the development of various cancers, including lung, prostate, and renal cancers. Notably, the primary substrates undergoing succinylation are non-histone proteins. Therefore, elucidating the functions of cancer-related succinylated proteins is essential for identifying novel therapeutic targets. This review comprehensively summarizes current research advances regarding protein succinylation in common cancers and discusses the progress in developing succinylation-targeting drugs. Specifically, we focus on the molecular mechanisms by which succinylation regulates cancer progression, along with the identification of key succinylation sites. Our discussion aims to provide valuable insights for future research and the development of innovative cancer treatments.
Keywords: cancer; drug; succinylation; tumor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

