Tumor-Specific EphA2 Receptor Tyrosine Kinase Inhibits Anti-Tumor Immunity by Recruiting Suppressive Myeloid Populations in Murine Models of Non-Small Cell Lung Cancer
- PMID: 40867322
- PMCID: PMC12384598
- DOI: 10.3390/cancers17162693
Tumor-Specific EphA2 Receptor Tyrosine Kinase Inhibits Anti-Tumor Immunity by Recruiting Suppressive Myeloid Populations in Murine Models of Non-Small Cell Lung Cancer
Abstract
Background: EphA2 is a receptor tyrosine kinase that contributes to tumor growth and metastasis and has been identified as a viable target for many solid cancers. Investigating EphA2's impact on the host immune system may advance our understanding of tumor immune evasion and the consequences of targeting EphA2 on the tumor microenvironment.
Methods: Here, we examine how tumor-specific EphA2 affects the activation and infiltration of immune cell populations and the cytokine and chemokine milieu in murine models of non-small cell lung cancer (NSCLC).
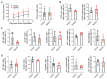
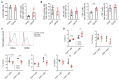
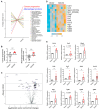
Results: Although EphA2 overexpression in NSCLC cells did not display proliferative advantage in vitro, it conferred a growth advantage in vivo. Analysis of lung tumor infiltrates via flow cytometry revealed decreased natural killer and T cells in the EphA2-overexpressing tumors, as well as increased myeloid populations, including tumor-associated macrophages (TAMs). T-cell activation, particularly in CD8+ T cells, was decreased, while PD-1 expression was increased. These changes were accompanied by increased monocyte-attracting chemokines, specifically CCL2, CCL7, CCL8, and CCL12, and immunosuppressive proteins TGF-β and arginase 1 in RNA expression analyses.
Conclusions: Our studies suggest EphA2 on tumor cells recruits monocytes and promotes their differentiation into TAMs that likely inhibit the activation and infiltration of cytotoxic lymphocytes, promoting tumor immune escape.
Keywords: EphA2; anti-tumor immunity; non-small cell lung cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

