Baseline Hemostatic Biomarker Assessment Identifies Breast Cancer Patients at High Risk for Venous Thromboembolism During Chemotherapy
- PMID: 40867341
- PMCID: PMC12384613
- DOI: 10.3390/cancers17162712
Baseline Hemostatic Biomarker Assessment Identifies Breast Cancer Patients at High Risk for Venous Thromboembolism During Chemotherapy
Abstract
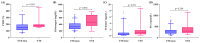
(1) Background: The presence of metastatic disease significantly increases the risk of venous thromboembolism (VTE) in breast cancer, particularly during chemotherapy. Although not categorized as a highly thrombogenic malignancy, the elevated global prevalence of this cancer places a substantial number of patients at risk of thrombosis, which cannot yet be accurately predicted by validated risk assessment models (RAMs), highlighting the need for a dedicated model. (2) Aim: This study aims to develop a RAM for VTE in newly diagnosed metastatic breast cancer patients enrolled in a prospective, observational, and multicenter study. (3) Methods: A cohort of 189 patients beginning antitumor therapy were enrolled and prospectively monitored for VTE and mortality. Blood samples collected at enrollment were tested for D-dimer, fibrinogen, FVIII, prothrombin fragment 1 + 2 (F1 + 2), and thrombin generation (TG). Competing risk analyses were performed to identify significant predictors. (4) Results: Within one year, the cumulative incidences of VTE and mortality were 7.0% and 12%, respectively. Univariable analysis identified high Ki-67, D-dimer, FVIII, fibrinogen, and TG levels, along with low hemoglobin levels, as independent predictors of VTE. Only Ki-67, fibrinogen, FVIII, and hemoglobin were retained as significant predictors in multivariable analysis. These variables were further examined by multiple linear regression, which revealed Ki-67 and fibrinogen as the most significant parameters. A continuous RAM was then developed based on Ki-67 and fibrinogen (c-statistics 0.78), categorizing patients into low-risk and high-risk groups for VTE (2% vs. 13%; SHR 3.6, p = 0.018). This stratification could not be achieved using currently validated models for VTE risk. (5) Conclusions: We developed an accurate RAM for VTE that enables the identification of metastatic breast cancer patients at high risk for VTE, which supports clinicians in personalized thromboprophylaxis strategies if externally validated.
Keywords: D-dimer; Ki-67; fibrinogen; hemostatic biomarkers; hypercoagulability; metastatic breast cancer; mortality; venous thromboembolism.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- ECIS—European Cancer Information System Breast Cancer in the EU. [(accessed on 7 August 2025)]. Available online: https://ecis.jrc.ec.europa.eu.
-
- Benitez Fuentes J.D., Morgan E., de Luna Aguilar A., Mafra A., Shah R., Giusti F., Vignat J., Znaor A., Musetti C., Yip C.H., et al. Global Stage Distribution of Breast Cancer at Diagnosis: A Systematic Review and Meta-Analysis. JAMA Oncol. 2024;10:71–78. doi: 10.1001/jamaoncol.2023.4837. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

