Extracellular Matrix (ECM) Aging in the Retina: The Role of Matrix Metalloproteinases (MMPs) in Bruch's Membrane Pathology and Age-Related Macular Degeneration (AMD)
- PMID: 40867504
- PMCID: PMC12383675
- DOI: 10.3390/biom15081059
Extracellular Matrix (ECM) Aging in the Retina: The Role of Matrix Metalloproteinases (MMPs) in Bruch's Membrane Pathology and Age-Related Macular Degeneration (AMD)
Abstract
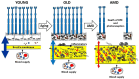
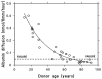
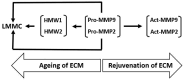
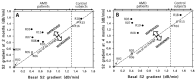
The extracellular matrix (ECM) is a collagen-based scaffold that provides structural support and regulates nutrient transport and cell signaling. ECM homeostasis depends on a dynamic balance between synthesis and degradation, the latter being primarily mediated by matrix metalloproteinases (MMPs). These enzymes are secreted as pro-forms and require activation to degrade ECM components. Their activity is modulated by tissue inhibitors of metalloproteinases (TIMPs). Aging disrupts this balance, leading to the accumulation of oxidized, cross-linked, and denatured matrix proteins, thereby impairing ECM function. Bruch's membrane, a penta-laminated ECM structure in the eye, plays a critical role in supporting photoreceptor and retinal pigment epithelium (RPE) health. Its age-related thickening and decreased permeability are associated with impaired nutrient delivery and waste removal, contributing to the pathogenesis of age-related macular degeneration (AMD). In AMD, MMP dysfunction is characterized by the reduced activation and sequestration of MMPs, which further limits matrix turnover. This narrative review explores the structural and functional changes in Bruch's membrane with aging, the role of MMPs in ECM degradation, and the relevance of these processes to AMD pathophysiology, highlighting emerging regulatory mechanisms and potential therapeutic targets.
Keywords: Bruch’s membrane; age-related macular degeneration (AMD); aging; extracellular matrix (ECM); matrix metalloproteinases (MMPs).
Conflict of interest statement
A.A.H. declares no conflicts of interest. Y.L. is employed by AltRegen Co., Ltd. The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
Bruch's membrane heparan sulfate retains lipoproteins in the early stages of age-related macular degeneration.Proc Natl Acad Sci U S A. 2025 Jun 17;122(24):e2500727122. doi: 10.1073/pnas.2500727122. Epub 2025 Jun 13. Proc Natl Acad Sci U S A. 2025. PMID: 40512794 Free PMC article.
-
Disturbed Matrix Metalloproteinases Activity in Age-Related Macular Degeneration.Adv Exp Med Biol. 2023;1415:21-26. doi: 10.1007/978-3-031-27681-1_4. Adv Exp Med Biol. 2023. PMID: 37440009 Review.
-
Potential Role of NUR77 in the Aging Retinal Pigment Epithelium and Age-Related Macular Degeneration.Adv Exp Med Biol. 2025;1468:165-169. doi: 10.1007/978-3-031-76550-6_27. Adv Exp Med Biol. 2025. PMID: 39930190 Review.
-
The Sialome of the Retina, Alteration in Age-Related Macular Degeneration Pathology, and Potential Impacts on Complement Factor H.Invest Ophthalmol Vis Sci. 2025 Jun 2;66(6):81. doi: 10.1167/iovs.66.6.81. Invest Ophthalmol Vis Sci. 2025. PMID: 40576434 Free PMC article.
-
Aberrant lipid accumulation and retinal pigment epithelium dysfunction in PRCD-deficient mice.Exp Eye Res. 2024 Sep;246:110016. doi: 10.1016/j.exer.2024.110016. Epub 2024 Aug 5. Exp Eye Res. 2024. PMID: 39098587 Free PMC article.
References
-
- Manou D., Caon I., Bouris P., Triantaphyllidou I.E., Giaroni C., Passi A., Karamanos N.K., Vigetti D., Theocharis A.D. The Extracellular Matrix: Methods and Protocols. Volume 1952. Humana Press; New York, NY, USA: 2019. The complex interplay between extracellular matrix and cells in tissues; pp. 1–20. Methods in Molecular Biology. - PubMed
-
- Theocharis A.D., Gialeli C., Hascall V.C., Karamanos N.K. Extracellular matrix: A functional scaffold. In: Karamanos N.K., editor. Extracellular Matrix: Pathobiology and Signalling. Walter de Gruyter GmbH & Co KG; Berlin, Germany: Boston, MA, USA: 2012. pp. 3–20.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

