A Distinct miRNA Profile in Intimal Hyperplasia of Failed Arteriovenous Fistulas Reveals Key Pathogenic Pathways
- PMID: 40867509
- PMCID: PMC12383664
- DOI: 10.3390/biom15081064
A Distinct miRNA Profile in Intimal Hyperplasia of Failed Arteriovenous Fistulas Reveals Key Pathogenic Pathways
Abstract
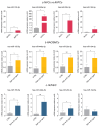
Intimal hyperplasia (IH) compromises the patency of arteriovenous fistula (AVF) vascular access in patients with end-stage kidney disease. Uncontrolled cell proliferation and migration, driven by inflammation, shear stress and surgery, are well-known triggers in IH. Recently, microRNAs (miRNAs) have emerged as regulators of core mechanisms in cardiovascular diseases and as potential markers of IH. This study was aimed at identifying a specific miRNA panel in failed AVFs and clarifying the miRNA involvement in IH. miRNA profiling performed in tissues from patients with IH (AVFs) and normal veins (NVs) highlighted a subset of four miRNAs significantly deregulated (hsa-miR-155-5p, hsa-miR-449a-5p, hsa-miR-29c-3p, hsa-miR-194-5p) between the two groups. These miRNAs were analyzed in tissue-derived cells (NVCs and AVFCs), human aortic smooth muscle cells (HAOSMCs) and human umbilical vein endothelial cells (HUVECs). The panel of hsa-miR-449a-5p, hsa-miR-155-5p, hsa-miR-29c-3p and hsa-miR-194-5p was up-regulated in AVFCs, HAOSMCs and HUVEC under inflammatory stimuli. Notably, overexpression of hsa-miR-449a-5p exacerbated the proliferative, migratory and inflammatory features of AVFCs. In vitro pharmacological modulation of these miRNAs with pioglitazone, particularly the down-regulation of hsa-miR-155-5p and hsa-miR-29c-3p, suggested their involvement in IH pathogenesis and a potential translational application. Overall, these findings provide new insights into the pathogenesis of AVF failure, reinforcing the miRNA contribution to IH detection and prevention.
Keywords: arteriovenous fistula failure; hsa-miR-449a-5p; intimal hyperplasia; miRNA; migration; proliferation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Martinez L., Tabbara M., Duque J.C., Selman G., Falcon N.S., Paez A., Griswold A.J., Ramos-Echazabal G., Hernandez D.R., Velazquez O.C., et al. Transcriptomics of Human Arteriovenous Fistula Failure: Genes Associated With Nonmaturation. Am. J. Kidney Dis. 2019;74:73–81. doi: 10.1053/j.ajkd.2018.12.035. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

