Hepatic Expression of ACBP Is a Prognostic Marker for Weight Loss After Bariatric Surgery
- PMID: 40867617
- PMCID: PMC12384758
- DOI: 10.3390/biom15081173
Hepatic Expression of ACBP Is a Prognostic Marker for Weight Loss After Bariatric Surgery
Abstract
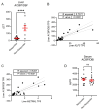
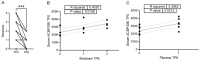
The incidence and prevalence of obesity and related cardio-metabolic diseases are on the rise, posing a critical health care challenge to systems across the globe. Bariatric surgery is a therapeutic cornerstone for morbidly obese patients, besides novel medical treatments, partly by ameliorating metabolic inflammation, a hallmark of metabolic diseases. Acyl-CoA Binding Protein (ACBP), also known as diazepam-binding inhibitor (DBI), is a regulator of autophagy and metabolism, and has recently been shown to increase in individuals undergoing voluntary fasting and in patients with cancer cachexia-induced malnutrition. By analyzing a prospectively collected study with matched serum and liver samples from patients undergoing laparoscopic adjustable gastric banding at baseline and six months after surgery, we here demonstrate that ACBP serum levels significantly increase following bariatric surgery. Hepatic ACBP expression at baseline predicted weight loss six months after the procedure. The predictive value of ACBP warrants further study, as it could identify patients who benefit most from metabolic surgery in the future.
Keywords: ACBP; DBI; bariatric surgery; weight loss.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet. 2024;403:1027–1050. doi: 10.1016/S0140-6736(23)02750-2. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

