Targeting HMGCS2: Ketogenesis Suppression Accelerates NAFLD Progression in T2DM Comorbidity, While Cynaroside Ameliorates NASH in Concomitant T2DM
- PMID: 40867627
- PMCID: PMC12385132
- DOI: 10.3390/biom15081181
Targeting HMGCS2: Ketogenesis Suppression Accelerates NAFLD Progression in T2DM Comorbidity, While Cynaroside Ameliorates NASH in Concomitant T2DM
Abstract
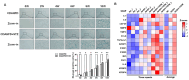
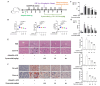
Patients with concurrent non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2DM) exhibit increased susceptibility to non-alcoholic steatohepatitis (NASH), advanced hepatic fibrosis, cirrhosis, and hepatocellular carcinoma. This study investigated the contribution of ketogenesis to T2DM-mediated NAFLD exacerbation and elucidated the therapeutic mechanism of cynaroside in NASH-complicated T2DM. Male C57BL/6J mice were given CDAHFD combined with streptozotocin to establish stage-specific NAFLD with T2DM models. Hepatic HMGCS2 expression was modulated via tail vein injection of adenoviral vectors for HMGCS2 overexpression or knockdown. Cynaroside was administered orally from week 5 to week 8. The results showed that concurrent T2DM accelerated NAFLD progression, accompanied by a dysregulated ketogenesis that was correlated with disease severity. Hepatic HMGCS2 expression paralleled circulating ketone body concentrations, indicating that HMGCS2-mediated ketogenic dysregulation contributed to NAFLD pathogenesis in T2DM contexts. HMGCS2 overexpression in NASH-T2DM models significantly attenuated steatohepatitis progression through the enhancement of ketogenesis. Cynaroside administration ameliorated hepatic pathology in NASH-T2DM mice by (1) reducing hepatocellular injury and lobular inflammation; (2) decreasing intrahepatic lipid accumulation; and (3) suppressing hepatocyte senescence and the secretion of SASP factors. Mechanistically, cynaroside exerted therapeutic effects via HMGCS2-mediated ketogenesis. Our data demonstrated that ketogenic modulation is a viable therapeutic strategy to delay T2DM-NAFLD progression.
Keywords: cellular senescence; cynaroside; hydroxymethylglutaryl-CoA synthase 2; ketogenesis; non-alcoholic fatty liver disease; type 2 diabetes mellitus.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures





Similar articles
-
Herbal mixture of Platycodon grandiflorum, Cinnamomum cassia, and Asiasarum sieboldii extracts protects against NASH progression via regulation of hepatic steatosis, inflammation, and apoptosis.Phytomedicine. 2025 Sep;145:157077. doi: 10.1016/j.phymed.2025.157077. Epub 2025 Jul 14. Phytomedicine. 2025. PMID: 40684491
-
Restoration of HMGCS2-mediated ketogenesis alleviates tacrolimus-induced hepatic lipid metabolism disorder.Acta Pharmacol Sin. 2024 Sep;45(9):1898-1911. doi: 10.1038/s41401-024-01300-0. Epub 2024 May 17. Acta Pharmacol Sin. 2024. PMID: 38760545 Free PMC article.
-
Rhein alleviates hepatic steatosis in NAFLD mice by activating the AMPK/ACC/SREBP1 pathway to enhance lipid metabolism.Mol Med. 2025 Jul 10;31(1):255. doi: 10.1186/s10020-025-01304-4. Mol Med. 2025. PMID: 40640749 Free PMC article.
-
Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis.Cochrane Database Syst Rev. 2013 Dec 27;2013(12):CD008623. doi: 10.1002/14651858.CD008623.pub2. Cochrane Database Syst Rev. 2013. PMID: 24374462 Free PMC article.
-
The Prevalence of Small Intestinal Bacterial Overgrowth in Patients with Non-Alcoholic Liver Diseases: NAFLD, NASH, Fibrosis, Cirrhosis-A Systematic Review, Meta-Analysis and Meta-Regression.Nutrients. 2022 Dec 9;14(24):5261. doi: 10.3390/nu14245261. Nutrients. 2022. PMID: 36558421 Free PMC article.
References
-
- En Li Cho E., Ang C.Z., Quek J., Fu C.E., Lim L.K.E., Heng Z.E.Q., Tan D.J.H., Lim W.H., Yong J.N., Zeng R., et al. Global prevalence of non-alcoholic fatty liver disease in type 2 diabetes mellitus: An updated systematic review and meta-analysis. Gut. 2023;72:2138–2148. doi: 10.1136/gutjnl-2023-330110. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 2023AH030105/the Excellent Young Scholar Projects of Natural Science Research Projects of Anhui Higher Edu-cation Institutions
- 2022AH040172/the major Project of Natural Science Research Projects of Anhui Higher Education Institutions
- 202410368045/the National College Students Innovation and Entrepreneurship Training Program
LinkOut - more resources
Full Text Sources
Medical

