Nicotinamide and Pyridoxine in Muscle Aging: Nutritional Regulation of Redox, Inflammation, and Regeneration
- PMID: 40867810
- PMCID: PMC12383212
- DOI: 10.3390/antiox14080911
Nicotinamide and Pyridoxine in Muscle Aging: Nutritional Regulation of Redox, Inflammation, and Regeneration
Abstract
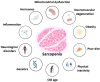
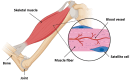
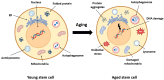
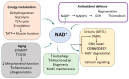
Sarcopenia, the progressive loss of muscle mass, strength, and regenerative capacity with age, is driven by interconnected processes such as oxidative stress, chronic inflammation, mitochondrial dysfunction, and reduced activity of muscle stem cells. As the population ages, nutritional strategies that target these mechanisms are becoming increasingly important. This review focuses on nicotinamide (vitamin B3) and pyridoxine (vitamin B6), two essential micronutrients found in functional foods, which play complementary roles in redox regulation, immune balance, and muscle repair. Nicotinamide supports nicotinamide adenine dinucleotide (NAD+) metabolism, boosts mitochondrial function, and activates sirtuin pathways involved in autophagy and stem cell maintenance. Pyridoxine, via its active form pyridoxal 5'-phosphate (PLP), is key to amino acid metabolism, antioxidant defense, and the regulation of inflammatory cytokines. We summarize how these vitamins influence major molecular pathways such as Sirtuin1 (SIRT1), protein kinase B (AKT)/mechanistic target of rapamycin (mTOR), Nuclear factor-κB (NF-κB), and Nrf2, contributing to improved myogenic differentiation and protection of the aging muscle environment. We also highlight emerging preclinical and clinical data, including studies suggesting possible synergy between B3 and B6. Finally, we discuss how biomarkers such as PLP, nicotinamide mononucleotide (NMN), and C-reactive protein (CRP) may support the development of personalized nutrition strategies using these vitamins. Safe, accessible, and mechanistically grounded, nicotinamide and pyridoxine offer promising tools for sarcopenia prevention and healthy aging.
Keywords: inflammaging; muscle regeneration; muscle stem cells; nicotinamide; nicotinamide adenine dinucleotide (NAD+) metabolism; nutritional intervention; pyridoxine; sarcopenia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
The effect of vitamin B6 on cognition.Cochrane Database Syst Rev. 2003;(4):CD004393. doi: 10.1002/14651858.CD004393. Cochrane Database Syst Rev. 2003. PMID: 14584010
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Small extracellular vesicles derived from mesenchymal stromal cells loaded with β-nicotinamide mononucleotide activate NAD+/SIRT3 signaling pathway-mediated mitochondrial autophagy to delay skin aging.Stem Cell Res Ther. 2025 Jul 1;16(1):339. doi: 10.1186/s13287-025-04460-w. Stem Cell Res Ther. 2025. PMID: 40598314 Free PMC article.
-
Umbelliferone attenuates diabetic sarcopenia by modulating mitochondrial quality and the ubiquitin-proteasome system.Phytomedicine. 2025 Aug;144:156930. doi: 10.1016/j.phymed.2025.156930. Epub 2025 May 31. Phytomedicine. 2025. PMID: 40483791
-
Therapeutic application of natural products: NAD+ metabolism as potential target.Phytomedicine. 2023 Jun;114:154768. doi: 10.1016/j.phymed.2023.154768. Epub 2023 Mar 13. Phytomedicine. 2023. PMID: 36948143 Review.
References
-
- Srivastava S., Pandey V.K., Singh A., Dar A.H. Exploring the Potential of Treating Sarcopenia through Dietary Interventions. J. Food Biochem. 2024;2024:3018760. doi: 10.1155/2024/3018760. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

