Melatonin During Pre-Maturation and Its Effects on Bovine Oocyte Competence
- PMID: 40867865
- PMCID: PMC12382782
- DOI: 10.3390/antiox14080969
Melatonin During Pre-Maturation and Its Effects on Bovine Oocyte Competence
Abstract
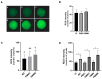
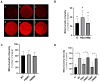
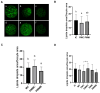
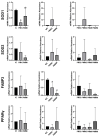
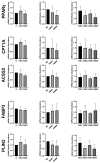
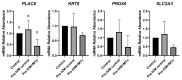
To minimize the deleterious effects of oxidative stress and improve oocyte competence, we assessed the impact of melatonin during in vitro pre-maturation (pre-IVM) in bovine cumulus-oocyte complexes (COCs). We compared three groups: control (conventional IVM), pre-IVM control (without melatonin), and pre-IVM + MTn (with melatonin). The analyses included levels of reactive oxygen species (ROS), mitochondrial activity, oocyte lipid content, and the expression of genes related to oxidative stress and lipid metabolism in oocytes and cumulus cells. We also examined embryo quality by evaluating kinetics of development and gene expression. The pre-IVM + MTn group exhibited an increase (p ≤ 0.05) in ROS levels and a decrease (p ≤ 0.05) in lipid content, while maintaining mitochondrial activity similar (p > 0.05) to that of the control group. Regarding gene expression, the effect of pre-IVM, independent of melatonin, was characterized by a decrease in FABP3 transcripts in cumulus cells and reductions in GSS and NFE2L2 transcripts in oocytes (p ≤ 0.05). The pre-IVM + MTn group also displayed a decrease (p ≤ 0.05) in CAT and SOD2 transcript levels. In terms of embryonic development, the pre-IVM + MTn group achieved a higher blastocyst rate on D7 (p ≤ 0.05) compared to the control group (30.8% versus 25.8%), but with similar rates (p > 0.05) to the pre-IVM control group (30.8% versus 35.9%). However, there was a decrease in the levels of the PLAC8 transcript. This study indicates that, under the conditions tested, melatonin did not significantly benefit oocyte competence.
Keywords: antioxidant; biotechniques; biotechnology; meiosis; qPCR.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

