An Update on Role of Ionizing Radiation to Enhance Proliferation and Differentiation of Normal Stem Cells via Activation of NRF2 Pathway: Review
- PMID: 40867882
- PMCID: PMC12382644
- DOI: 10.3390/antiox14080986
An Update on Role of Ionizing Radiation to Enhance Proliferation and Differentiation of Normal Stem Cells via Activation of NRF2 Pathway: Review
Abstract
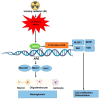
Ionizing radiation (IR) as a stress inducer has a significant impact on various normal stem cells differentiation through activation of various signaling pathways. Low levels of oxidative stress of IR may preserve or even enhance cell differentiation. In response to IR, reactive oxygen species (ROS) can activate various signaling pathways that promote cell differentiation, notably through the involvement of nuclear factor erythroid 2-related factor 2 (NRF2). NRF2 interacts with multiple pathways, including Wnt/β-catenin (osteogenesis), PPARγ (adipogenesis), and BDNF/TrkB (neurogenesis). This response is dose-dependent: low doses of IR activate NRF2 and support differentiation, while high doses can overwhelm the antioxidant system, resulting in cell death. However, the quality of various types of IR, such as proton and carbon ion radiation, may have a varied impact on stem cells (SCs) differentiation compared to X-rays. Hence, activation of the NRF2 signaling pathway in SCs and cell differentiation depends on the level of stress and the quality and quantity of IR. This review is an update to explore how IR modulates SCs fate toward osteogenic, adipogenic, and neurogenic lineages through the NRF2 signaling pathway. We highlight mechanistic insights, dose-dependent effects, and therapeutic implications, bridging gaps between experimental models and clinical translation.
Keywords: NRF2; PI3K/Ak; differentiation; ionizing radiation; neurogenesis; osteogenesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Li Y., Yue G., Yu S., Liu Z., Cao Y., Wang X. Extracellular Vesicles Derived from H2O2-Stimulated Adipose-Derived Stem Cells Alleviate Senescence in Diabetic Bone Marrow Mesenchymal Stem Cells and Restore Their Osteogenic Capacity. Drug Des. Dev. Ther. 2024;18:2103–2124. doi: 10.2147/DDDT.S454509. - DOI - PMC - PubMed
-
- Carrière A., Ebrahimian T.G., Dehez S., Augeé N., Joffre C., Andreé M., Arnal S., Duriez M., Barreau C., Arnaud E. Preconditioning by mitochondrial reactive oxygen species improves the proangiogenic potential of adipose-derived cells-based therapy. Arterioscler. Thromb. Vasc. Biol. 2009;29:1093–1099. doi: 10.1161/ATVBAHA.109.188318. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

