Living on the Edge: ROS Homeostasis in Cancer Cells and Its Potential as a Therapeutic Target
- PMID: 40867898
- PMCID: PMC12382852
- DOI: 10.3390/antiox14081002
Living on the Edge: ROS Homeostasis in Cancer Cells and Its Potential as a Therapeutic Target
Abstract
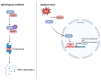
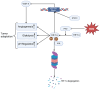
Reactive oxygen species (ROS) act as double-edged swords in cancer biology-facilitating tumor growth, survival, and metastasis at moderate levels while inducing oxidative damage and cell death when exceeding cellular buffering capacity. To survive under chronic oxidative stress, cancer cells rely on robust antioxidant systems such as the glutathione (GSH) and thioredoxin (Trx), and superoxide dismutases (SODs). These systems maintain redox homeostasis and sustain ROS-sensitive signaling pathways including MAPK/ERK, PI3K/Akt/mTOR, NF-κB, STAT3, and HIF-1α. Targeting the antioxidant defense mechanisms of cancer cells has emerged as a promising therapeutic strategy. Inhibiting the glutathione system induces ferroptosis, a non-apoptotic form of cell death driven by lipid peroxidation, with compounds like withaferin A and altretamine showing strong preclinical activity. Disruption of the Trx system by agents such as PX-12 and dimethyl fumarate (DMF) impairs redox-sensitive survival signaling. Trx reductase inhibition by auranofin or mitomycin C further destabilizes redox balance, promoting mitochondrial dysfunction and apoptosis. SOD1 inhibitors, including ATN-224 and disulfiram, selectively enhance oxidative stress in tumor cells and are currently being tested in clinical trials. Mounting preclinical and clinical evidence supports redox modulation as a cancer-selective vulnerability. Pharmacologically tipping the redox balance beyond the threshold of cellular tolerance offers a rational and potentially powerful approach to eliminate malignant cells while sparing healthy tissue, highlighting novel strategies for targeted cancer therapy at the interface of redox biology and oncology.
Keywords: antioxidant defense; cancer metabolism; cancer therapy; reactive oxygen species (ROS); redox signaling.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Reactive Oxygen Species: A Double-Edged Sword in the Modulation of Cancer Signaling Pathway Dynamics.Cells. 2025 Aug 6;14(15):1207. doi: 10.3390/cells14151207. Cells. 2025. PMID: 40801639 Free PMC article. Review.
-
A systematic review of p53 regulation of oxidative stress in skeletal muscle.Redox Rep. 2018 Dec;23(1):100-117. doi: 10.1080/13510002.2017.1416773. Epub 2018 Jan 3. Redox Rep. 2018. PMID: 29298131 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
The Redox Revolution in Brain Medicine: Targeting Oxidative Stress with AI, Multi-Omics and Mitochondrial Therapies for the Precision Eradication of Neurodegeneration.Int J Mol Sci. 2025 Aug 3;26(15):7498. doi: 10.3390/ijms26157498. Int J Mol Sci. 2025. PMID: 40806624 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

