Cucurbitacin E Suppresses Adipogenesis and Lipid Accumulation in 3T3-L1 Adipocytes Without Cytotoxicity
- PMID: 40868081
- PMCID: PMC12383856
- DOI: 10.3390/biomedicines13081826
Cucurbitacin E Suppresses Adipogenesis and Lipid Accumulation in 3T3-L1 Adipocytes Without Cytotoxicity
Abstract
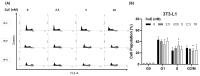
Background: Cucurbitacin E (CuE), a natural tetracyclic triterpenoid compound extracted from the melon stems of Cucurbitaceae plants, has been reported to exhibit anti-inflammatory and anti-cancer properties, along with the ability to enhance cellular immunity. However, its role and molecular mechanism in regulating lipid metabolism and adipogenesis remain unclear. This study aims to investigate the potential anti-adipogenic and anti-obesity effects of CuE in 3T3-L1 adipocytes. Materials and Methods: 3T3-L1 preadipocytes were cultured and induced to differentiate using a standard adipogenic cocktail containing dexamethasone, 3-isobutyl-1-methylxanthine (IBMX), and insulin (DMI). CuE was administered during the differentiation process at various concentrations. Lipid accumulation was assessed using Oil Red O staining, and cell viability was evaluated via the MTT assay. To determine whether CuE induced apoptosis or necrosis, flow cytometry was performed using annexin V/PI staining. Additional molecular analyses, such as Western blotting and RT-PCR, were used to examine the expression of key adipogenic markers. Results: Treatment with CuE significantly reduced lipid droplet formation in DMI-induced 3T3-L1 adipocytes in a dose-dependent manner, as shown by decreased Oil Red O staining. Importantly, CuE did not induce apoptosis or necrosis in 3T3-L1 cells at effective concentrations, indicating its safety toward normal adipocytes. Moreover, CuE treatment downregulated the expression of adipogenic markers such as PPARγ and C/EBPα at both mRNA and protein levels. Discussion: Our findings suggest that CuE exerts a non-cytotoxic inhibitory effect on adipocyte differentiation and lipid accumulation. This anti-adipogenic effect is likely mediated through the suppression of key transcription factors involved in adipogenesis. The absence of cytotoxicity supports the potential application of CuE as a safe bioactive compound for obesity management. Further investigation is warranted to elucidate the upstream signaling pathways and in vivo efficacy of CuE. Conclusions: Cucurbitacin E effectively inhibits adipogenesis in 3T3-L1 adipocytes without inducing cytotoxic effects, making it a promising candidate for the development of functional foods or therapeutic agents aimed at preventing or treating obesity. This study provides new insights into the molecular basis of CuE's anti-obesity action and highlights its potential as a natural lipogenesis inhibitor.
Keywords: 3T3-L1 cells; Anti-Obesity Medication (AOM); adipocyte differentiation; cucurbitacin E; obesity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Effects of 23-epi-26-deoxyactein on adipogenesis in 3T3-L1 preadipocytes and diet-induced obesity in C57BL/6 mice.Phytomedicine. 2020 Sep;76:153264. doi: 10.1016/j.phymed.2020.153264. Epub 2020 Jun 5. Phytomedicine. 2020. PMID: 32570112
-
Triclosan (TCS) promotes lipid accumulation in the mouse adipocyte (3T3-L1) cell line via peroxisome proliferator activated receptor gamma (PPARγ) pathway.Toxicol Appl Pharmacol. 2025 Oct;503:117482. doi: 10.1016/j.taap.2025.117482. Epub 2025 Jul 23. Toxicol Appl Pharmacol. 2025. PMID: 40712680
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

