Protein Expression of TXNIP in the Dopaminergic Neurons of Subjects with Parkinson's Disease: Evidence from a Pilot Study
- PMID: 40868900
- PMCID: PMC12387627
- DOI: 10.3390/life15081252
Protein Expression of TXNIP in the Dopaminergic Neurons of Subjects with Parkinson's Disease: Evidence from a Pilot Study
Abstract
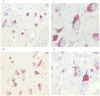
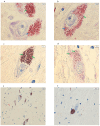
Parkinson's disease (PD) is a progressive, multisystemic α-synucleinopathy, recognized as the second most prevalent neurodegenerative disorder globally. Its neuropathology is characterized by the degeneration of dopaminergic neurons, particularly in the substantia nigra pars compacta (SNpc), and the intraneuronal accumulation of α-synuclein-forming Lewy bodies. Oxidative stress is a key contributor to PD pathogenesis. Thioredoxin-interacting protein (TXNIP) is a crucial regulator of cellular redox balance, inhibiting the antioxidant function of thioredoxin. This pilot study aimed to investigate the protein expression and localization of TXNIP in the SNpc of PD patients compared to healthy controls. We performed immunohistochemical analyses on 12 post-mortem human brain sections (formalin-fixed, paraffin-embedded) from six subjects with PD and six healthy controls. The study was performed on PD subjects with Braak stage 6. Our findings revealed that in control samples, TXNIP protein was distinctly and closely associated with neuromelanin (NM) pigment within the cytoplasm of SNpc dopaminergic neurons. Conversely, in PD samples, there was a markedly weak cytoplasmic expression of TXNIP, and critically, this association with NM pigment was absent. Furthermore, PD samples exhibited a significant reduction in both dopaminergic neurons and NM content, consistent with advanced disease. These findings, which mirror previous transcriptomic data showing TXNIP gene under-expression in the same subjects, suggest that altered TXNIP expression and localization in SNpc dopaminergic neurons are features of late-stage PD, potentially reflecting neuronal dysfunction and loss.
Keywords: Parkinson’s disease; TXNIP protein; dopaminergic neurons; immunohistochemistry; substantia nigra.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Decreased neuronal and increased endothelial fractalkine expression are associated with neuroinflammation in Parkinson's disease and related disorders.Front Cell Neurosci. 2025 Aug 6;19:1557645. doi: 10.3389/fncel.2025.1557645. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40842561 Free PMC article.
-
Tissue Factor and Its Cerebrospinal Fluid Protein Profiles in Parkinson's Disease.J Parkinsons Dis. 2024;14(7):1405-1416. doi: 10.3233/JPD-240115. J Parkinsons Dis. 2024. PMID: 39240648 Free PMC article.
-
Parkinson's Paradox: Alpha-synuclein's Selective Strike on SNc Dopamine Neurons over VTA.bioRxiv [Preprint]. 2025 Apr 2:2025.03.24.644952. doi: 10.1101/2025.03.24.644952. bioRxiv. 2025. Update in: NPJ Parkinsons Dis. 2025 Jul 11;11(1):207. doi: 10.1038/s41531-025-01055-3. PMID: 40236072 Free PMC article. Updated. Preprint.
-
Berberine's paradox in Neurodegeneration: Therapeutic promise and safety challenges in Parkinson's disease.Neuropharmacology. 2025 Nov 1;278:110555. doi: 10.1016/j.neuropharm.2025.110555. Epub 2025 Jun 6. Neuropharmacology. 2025. PMID: 40482960 Review.
-
The Search for Disease Modification in Parkinson's Disease-A Review of the Literature.Life (Basel). 2025 Jul 23;15(8):1169. doi: 10.3390/life15081169. Life (Basel). 2025. PMID: 40868817 Free PMC article. Review.
References
-
- Fisicaro F., Lanza G., Cantone M., Ferri R., Pennisi G., Nicoletti A., Zappia M., Bella R., Pennisi M. Clinical and Electrophysiological Hints to TMS in De Novo Patients with Parkinson’s Disease and Progressive Supranuclear Palsy. J. Pers. Med. 2020;10:274. doi: 10.3390/jpm10040274. - DOI - PMC - PubMed
-
- Checkoway H., Lundin J.I., Kelada S.N. Neurodegenerative Diseases. IARC Sci. Publ. 2011;163:407–419. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

