Cytotoxic Effects of Thymus serpyllum L. and Mentha × piperita L. Essential Oils on Basal Cell Carcinoma-An In Vitro Study
- PMID: 40868944
- PMCID: PMC12387631
- DOI: 10.3390/life15081296
Cytotoxic Effects of Thymus serpyllum L. and Mentha × piperita L. Essential Oils on Basal Cell Carcinoma-An In Vitro Study
Abstract
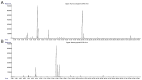
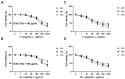
This study investigated the potential of Thymus serpyllum L. and Mentha × piperita L. essential oils (EOs), known for their bioactive properties, as adjunctive treatments targeting Basal cell carcinoma cancer stem cells (BCC CSCs). Primary cultures were established from ten BCC tumor samples and their distant resection margins as controls. The chemical composition of the EOs was analyzed by gas chromatography-mass spectroscopy (GC-MS) and attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR). The biological effects were evaluated via colony and spheroid formation, scratch assays, MTT and neutral red cytotoxicity assays, and qRT-PCR for Hh (SHH, PTCH1, SMO, and GLI1) and Notch (Notch1 and JAG1) gene expression. GC analysis identified thymol, p-cymene, and linalool as the main components of the EO of T. serpyllum L., and menthone and menthol in the EO of M. × piperita L. IC50 values were 262 µg/mL for T. serpyllum L. and 556 µg/mL for M. × piperita L. and were applied in all experiments. Both EOs significantly reduced CSC clonogenicity and migration (p < 0.05). The EO of T. serpyllum L. downregulated SMO and GLI1, while the EO of M. × piperita L. upregulated PTCH1, Notch1, and JAG1 (p < 0.05). These findings suggest that both EOs exhibit anticancer effects in BCC CSCs by modulating key oncogenic pathways, supporting their potential in BCC therapy.
Keywords: Mentha × piperita L.; Thymus serpyllum L.; basal cell carcinoma; cancer stem cells; essential oil.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Chemical Composition, Antioxidant Activity, and Multivariate Analysis of Four Moroccan Essential Oils: Mentha piperita, Mentha pulegium, Thymus serpyllum, and Thymus zygis.ScientificWorldJournal. 2024 Nov 27;2024:5552496. doi: 10.1155/tswj/5552496. eCollection 2024. ScientificWorldJournal. 2024. PMID: 39633960 Free PMC article.
-
Essential oils from Eucalyptus camaldulensis, Mentha piperita L., and Rosmarinus officinalis L.: a new frontier in combating antibiotic-resistant E. coli by inducing cellular components release.Braz J Microbiol. 2025 Jun;56(2):1155-1167. doi: 10.1007/s42770-025-01664-3. Epub 2025 Apr 16. Braz J Microbiol. 2025. PMID: 40237922
-
Enhanced antimicrobial and biofilm disruption efficacy of the encapsulated Thymus pallidus and Lavandula stoechas essential oils and their mixture: A synergistic approach.Int J Pharm. 2025 Feb 10;670:125144. doi: 10.1016/j.ijpharm.2024.125144. Epub 2024 Dec 27. Int J Pharm. 2025. PMID: 39734057
-
Sun protection for preventing basal cell and squamous cell skin cancers.Cochrane Database Syst Rev. 2016 Jul 25;7(7):CD011161. doi: 10.1002/14651858.CD011161.pub2. Cochrane Database Syst Rev. 2016. PMID: 27455163 Free PMC article.
-
A comprehensive review on traditional applications, phytochemistry, pharmacology, and toxicology of Thymus serpyllum.Indian J Pharmacol. 2023 Nov-Dec;55(6):385-394. doi: 10.4103/ijp.ijp_220_22. Indian J Pharmacol. 2023. PMID: 38174535 Free PMC article. Review.
References
-
- Global Burden of Disease 2019 Cancer Collaboration. Kocarnik J.M., Compton K., Dean F.E., Fu W., Gaw B.L., Harvey J.D., Henrikson H.J., Lu D., Pennini A., et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022;8:420–444. doi: 10.1001/jamaoncol.2021.6987. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

