Short-term effects of DAPAgliflozin on Lung fUNction, sleep apneas, and circulatinG surfactant protein B in Heart Failure with reduced ejection fraction (DAPA-LUNG-HF)
- PMID: 40869016
- PMCID: PMC12386758
- DOI: 10.3390/ijms26167696
Short-term effects of DAPAgliflozin on Lung fUNction, sleep apneas, and circulatinG surfactant protein B in Heart Failure with reduced ejection fraction (DAPA-LUNG-HF)
Abstract
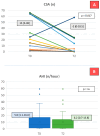
The mechanisms underlying the effects of dapagliflozin in heart failure with reduced ejection fraction (HFrEF) are not yet fully understood. This study aims to evaluate the effect of the drug on cardiorespiratory function by assessing alveolar-capillary membrane characteristics, sleep apnea, pulmonary and cardiac performance in stable HFrEF patients. Seventy-three patients with stable HFrEF were enrolled, with 66 completing the six-month follow-up. Analyses included assessment of the alveolar-capillary membrane by diffusion capacity, including its membrane diffusion and capillary volume components and measurements of proSP-B in the blood, an emerging biomarker of alveolar-capillary membrane function. Pulmonary function tests, overnight respiratory monitoring, and echocardiographic parameter collection were also conducted. After 6 months, a reduction in circulating proSP-B levels was observed (32.65 ± 13.36 at baseline vs. 30.86 ± 12.45 AU at 6 months, p for trend 0.0092), accompanied by improvements in echocardiographic parameters (left ventricle ejection fraction and pulmonary pressures). Pulmonary function tests and overnight respiratory monitoring showed no significant changes in lung diffusion, spirometry, or obstructive sleep apnea (apnea hypopnea index from 5.0 [1.1-16.6] at baseline to 6.2 [0.7-13.8]/h; p = n.s.). A significant reduction in central sleep apnea (CSA) was noted in the 13 patients with at least one CSA at baseline (15 [3-48] vs. 0 [0-18.5]/h, p = 0.017). Dapagliflozin demonstrates both hemodynamic and non-hemodynamic effects, particularly improving alveolar-capillary membrane function. This study highlights the multifactorial benefits of dapagliflozin in patients with stable HFrEF and the potential of proSP-B as a sensitive marker for evaluating therapeutic response.
Keywords: HFrEF; SGLT2i; dapagliflozin; lung diffusion; sleep apneas; surfactant binding proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- McMurray J.J.V., Solomon S.D., Inzucchi S.E., Kober L., Kosiborod M.N., Martinez F.A., Ponikowski P., Sabatine M.S., Anand I.S., Belohlavek J., et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. - DOI - PubMed
-
- McDonald M., Virani S., Chan M., Ducharme A., Ezekowitz J.A., Giannetti N., Heckman G.A., Howlett J.G., Koshman S.L., Lepage S., et al. CCS/CHFS Heart Failure Guidelines Update: Defining a New Pharmacologic Standard of Care for Heart Failure With Reduced Ejection Fraction. Can. J. Cardiol. 2021;37:531–546. doi: 10.1016/j.cjca.2021.01.017. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

