In Vitro Modeling of Diurnal Changes in Bone Metabolism
- PMID: 40869020
- PMCID: PMC12386813
- DOI: 10.3390/ijms26167699
In Vitro Modeling of Diurnal Changes in Bone Metabolism
Abstract
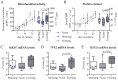
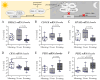
There is evidence that bone health is closely linked to a functioning circadian rhythm. Most of the evidence comes from mice, which may exhibit some species-specific differences from humans due to their nocturnal lifestyle. To address the current lack of human model systems, the present study aimed to develop an in vitro model system that can represent diurnal changes in bone metabolism. The model is based on co-cultured SCP-1 and THP-1 cells that serve as osteoblast and osteoclast precursors, respectively. Diurnal effects were induced by replacing the FCS in the differentiation medium with human serum pools (HSPs) obtained in the morning, noon, or evening. The model system was tested for cell viability, gene expression, and osteoblast and osteoclast function. The replacement of the FCS with the HSPs increased viability and induced expression changes in circadian clock genes in the model system. Resulting alterations in osteoblast and osteoclast function led to a gradual increase in mineral density and stiffness when 3D co-cultures were differentiated in the presence of the HSPs collected in the morning, noon, or evening, respectively. Here, we present for the first time an in vitro model that can present diurnal changes in bone metabolism in the form of a snapshot. With the simple use of HSPs, this model can be used as a platform technique to investigate bone function in various situations, taking into account the time of day.
Keywords: BMAL1; CLOCK; circadian rhythm; in vitro model; osteoblast; osteoclast.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

